Reports: UNI355342-UNI3: Catalytic Hydrogenations Using First Row Transition Metals
William McNamara, College of William and Mary
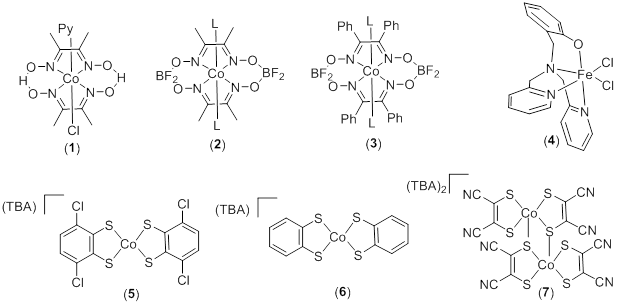
During our first two years of funding, we have synthesized the complexes represented in Figure 1. These compounds represent a series of cobalt and iron hydrogen generation catalysts that operate at various redox potentials. With a diverse range of electrochemical potentials for hydride formation, this library of catalysts presents a very interesting group of complexes to examine for catalytic hydrogen transfer.
Figure 1. Representative Complexes Examined for Transfer Hydrogenation
These complexes were synthesized by three undergraduate researchers and fully characterized using NMR, ESI-MS, and X-ray crystallography (for new compounds). When using isopropanol as the hydrogen source, all complexes in Figure 1 demonstrated modest activity (at best) for transfer hydrogenation of activated substrates. The substrates examined included methyl cinnamate, benzoquinone, acetophenone, and norbornene. Of these complexes, 4 showed the highest activity for the transfer hydrogenation of benzophenone (57% conversion, 200 turnovers). Although conversion was observed, these iron catalysts do not compare favorably to noble metal catalysts that have been previously reported. We then synthesized cobalt complexes with amine ligands that may act as proton relays in hydrogenation reactions (Figure 2). Unfortunately, we found that complex 4 was the most active catalyst for all substrates tested. Ongoing research focuses on the incorporation of amine functional groups near the active site of these catalysts.
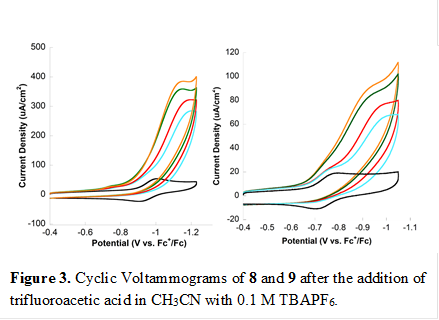
 Complexes 8 and 9 where synthesized and fully characterized for use in transfer hydrogenation catalysis. We reasoned that the amine functional groups bonded to cobalt could act as proton relays for transfer hydrogenation. Unfortunately, these complexes were not active for hydrogen transfer catalysis. We then tested their applicability of these complexes for proton reduction catalysis. We found that 8 and 9 show catalytic activity at -0.96 V and -1.1 V vs Fc+/Fc (Figure 3), respectively, resulting in overpotentials of 120 mV and 280 mV. Foot-of-the-wave analysis is used to examine the kinetic properties of these complexes, yielding a theoretical TOFmax of up to 4,100 s-1. Experimental TOFs of 7.1 s-1and 2.8 s-1 were also observed.
Complexes 8 and 9 where synthesized and fully characterized for use in transfer hydrogenation catalysis. We reasoned that the amine functional groups bonded to cobalt could act as proton relays for transfer hydrogenation. Unfortunately, these complexes were not active for hydrogen transfer catalysis. We then tested their applicability of these complexes for proton reduction catalysis. We found that 8 and 9 show catalytic activity at -0.96 V and -1.1 V vs Fc+/Fc (Figure 3), respectively, resulting in overpotentials of 120 mV and 280 mV. Foot-of-the-wave analysis is used to examine the kinetic properties of these complexes, yielding a theoretical TOFmax of up to 4,100 s-1. Experimental TOFs of 7.1 s-1and 2.8 s-1 were also observed.
We have also recently synthesized two Fe(II) complexes, 10 and 11, that are currently under investigation for use in transfer hydrogenation catalysis. These complexes are also being examined for electrocatalytic H2 activation, which may provide insight into the mechanism for hydrogenation. The full characterization of these complexes is currently underway and we anticipate that H2 activation and hydrogenation experiments will be complete during the final year of this grant.
Impact
This ACS-PRF grant provided the necessary funding to jumpstart our project on transfer hydrogenation chemistry using first-row metal complexes. It has allowed my group to purchase the materials needed to conduct this research. Furthermore, this grant has provided support for one undergraduate summer students (Brett Barden) to conduct research in this area during the summer of 2017. Another student (Zachary Schiffman) was not funded directly by the grant, but worked during the summer of 2017 on this project. An oral presentation on this research was presented by the PI at the 254th National ACS Meeting in Washington, DC during the summer of 2017. The PI will also give an oral presentation of this research at the 255th National ACS Meeting in New Orleans, LA. Two undergraduate students also presented posters at the 254th National ACS meeting in Washington, DC. The research experience helped the summer students learn critical thinking skills as well as specific chemical techniques such as NMR, IR, UV-Vis, electrochemistry, and X-ray crystallography. Both Brett Barden and Shichuan Xi (funded during summer of 2016) are currently applying to graduate schools to pursue a PhD in chemistry upon graduation in the spring. During the second year of funding, our work has been published in Inorganic Chemistry and Dalton  Transactions
Transactions
Future Directions
Our group is currently synthesizing variations of our iron catalysts that contain amine functional groups that are in close proximity to the active site of the catalyst (complex 10). Pendant amines have been found to increase the activity of certain ruthenium catalysts for transfer hydrogenation. Several students are also examining the use of other proton relays near that active site such as carboxylic acids. Once synthesized, these catalysts will be used for transfer hydrogenation catalysis of ketones and alkenes. H2 oxidation using these complexes will also be examined using electrochemical techniques.