Reports: ND154823-ND1: Acid Catalyzed Alkylation Reactions of Trichloroacetimidates
John D. Chisholm, Syracuse University
Overview
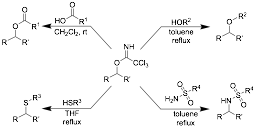
Trichloroacetimidates are known to be excellent alkylating agents when activated by a catalytic amount of a Brønsted or Lewis acid. Initially, this PRF ND-supported research was focused on evaluating a number of nucleophiles in direct alkylation reactions without the addition of an acid catalyst (Scheme 1). These studies showed that many imidates are much more reactive than predicted, with carboxylic acids, alcohols, thiols and sulfonamides being alkylated by the imidates without the need for a catalyst. Current projects are focused on the alkylation of indoles with trichloroacetimidates and the rearrangement of benzylic imidates to the corresponding benzylic acetamides.
Scheme 1. Substitution Reactions of Trichloroacetimidates without Exogenous Acid Catalysts
Exploring Indole Alkylations with Trichloroacetimidates
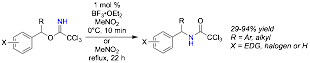
The displacement of trichloroacetimidates with carbon nucleophiles are now being explored. Initially, indoles were chosen as nucleophiles as these substrates are present in many important systems. Trichloroacetimidates function as effective electrophiles for the selective C3-alkylation of 2,3-disubstituted indoles to provide 3,3′- disubstituted indolenines (Scheme 2). Effective reaction conditions utilizing Lewis acid catalysts have been determined, and the scope of the reaction with respect to indole and imidate reaction partner has been investigated. This chemistry provides an alternative to base promoted and transition metal catalyzed methods that are more commonly utilized to access similar indolenines. This dearomatization of indoles is a powerful strategy for the synthesis of 3,3’-disubstituted indolenine intermediates, with similar indolenine substructures being present in many complex molecules.
Scheme 2. Alkylation of 2,3-Disubtituted Indoles with Trichloroacetimidates
Rearrangement of Benzylic Trichloroacetimidates to Benzylic Trichloroacetamides
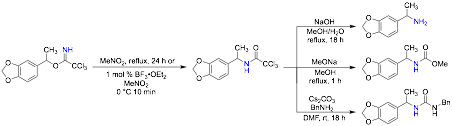
The rearrangement of allylic trichloroacetimidates is often used to access allylic amines, but the corresponding rearrangement of benzylic trichloroacetimidates has not been explored to synthesize benzylic amines. Conditions that provide the trichloroacetamide product from a benzylic trichloroacetimidate in high yield have also been developed (Scheme 3). A cationic mechanism for the rearrangement is implicated by the available data.
Scheme 3. Rearrangement of Benzylic Trichloroacetimidates to Benzylic Trichloroacetamides
Methods were also investigated to transform the trichloroacetamide product directly into the corresponding amine, carbamate, and urea (Scheme 4). Currently conditions for an enantioselective rearrangement are being pursued.
Scheme 4. Rearrangement of an Arylethyl Imidate and Elaboration of the Acetamide Product
Impact of PRF ND Award
In addition to providing several new methods for the formation of carbon-carbon and carbon-heteroatom bonds, this award has provided for the training of undergraduate and graduate students in the field of synthetic organic chemistry. These researchers were intimately involved in developing the new chemical methods and utilizing organic chemistry to synthesize substrates for the new reactions. By developing new methods and pursuing synthetic goals, students profit by learning how to solve problems in organic chemistry. In addition these experiences cultivate creativity, independence and resourcefulness. Other training activities for students include formal research presentations at meetings like National meetings of the American Chemical Society, Regional meetings of the American Chemical Society, the National Organic Symposium, Meetings of the NY Academy of Science and Gordon Conferences.