Reports: ND556136-ND5: Fundamental Insights into Heterogeneously Catalyzed Reactions Using Modulation Excitation FT-IR and Raman Spectroscopy
Ive Hermans, University of Wisconsin, Madison
The aim of this research proposal is to gain insight into the fundamental nature of heterogeneous catalysts by using operando Modulation Excitation Spectroscopy Diffuse Reflectance InfraRed Fourier Transform Spectroscopy (MES-DRIFTS). Due to the complex processes that occur on a heterogeneous catalyst surface under reaction conditions, IR spectroscopy usually becomes difficult to interpret. The state-of-the-art MES technique allows us to separate the active species from the spectator species, and even to enhance the signal-to-noise ratio. Moreover, MES can also give semi-quantitative micro-kinetic information. Here, we use operando MES-DRIFTS to investigate the influence of metal doping on the Lewis acid catalyst, Ag-Zr-BEA, for the production of 1,3-butadiene directly from ethanol.
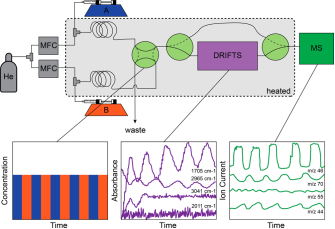
In the initial phase of the project, we constructed an operando MES-DRIFTS setup whereby we interfaced a mass spectrometer to the DRIFTS cell to collect kinetic data. To perform MES-DRIFTS experiments using a liquid substrate, we use syringe pumps, injecting the liquid substrate into a heated stainless spiral with flowing inert carrier gas which stabilizes the gas flow gained from vaporized liquid substrates (Figure 1). Also, by using 2-position 4-way valves, we can generate two different gas flows to operate modulation. Our setup also allows us to direct gas flow to the exhaust line until it reaches a steady concentration. After steady conditions are reached, we direct the gas flow to DRIFTS cell, which contains the catalyst, and switch between these two channels periodically.
Figure 1: Schematic diagram for MES-DRIFTS-MS setup for liquid substrates. The syringe pump injects the liquid into an inert carrier gas flow in a heated coil and, by using 2-position 4-way valves, two different compositions of gas flow can be modulated between two channels. The DRIFTS cell can be interfaced to a MS to collect kinetic data.
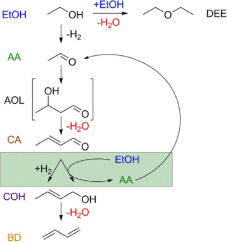
We studied the reaction mechanism for the production of 1,3-butadiene (BD) from ethanol/acetaldehyde (EtOH/AA) coupling over Ta-BEA catalyst using MES-DRIFTS. The reaction scheme for the production of 1,3-butadiene from ethanol is shown in Figure 2. We showed the productivity of BD is sensitive to the ratio of EtOH/AA. In the first step, crotonaldehyde (CA) will form by the aldol condensation of AA and EtOH, and then reduced with EtOH through a Meerwein-Ponndorf-Verley (MPV) mechanism to form crotyl alcohol (COH), which can then dehydrate to form BD. However, the disadvantage of this approach is that AA has to be co-fed with EtOH to complete the catalytic process. Since the Lewis acid sites are not able to dehydrogenate the EtOH to AA, we doped Ag to add dehydrogenation functionality, which can enhance the activity and selectivity.
Figure 2: Schematic reaction mechanism for synthesizing 1,3-butdiene from ethanol coupling.
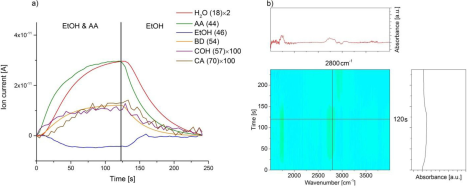
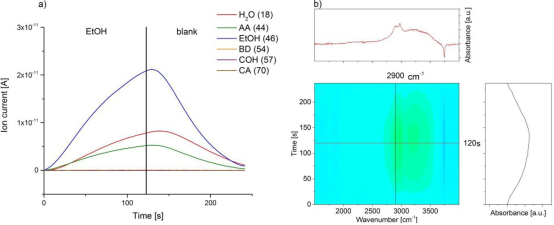
The MES-DRIFTS experiments performed using Zr-BEA as a catalyst (Figure 3) indicated that the peak corresponding to the carbonyl group of CA was shifted to 1693 cm-1 from 1710 cm-1 due to coordination of C=O to Zr Lewis acid sites. The desorption or consumption of EtOH and COH can be observed as decreasing C-H stretching at 2900 cm-1 in the first period. Overall, the behavior of Zr-BEA has a similar pattern in producing BD from EtOH and AA mixture with Ta-BEA.
Figure 3: Modulation experiment between EtOH/AA (3:1) and EtOH in helium flow over Zr-BEA at 320oC. (a) Recorded MS spectra and (B) diffuse reflectance FT-IR spectra.
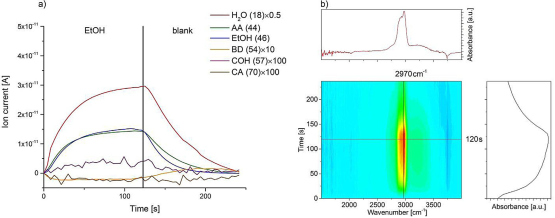
When we doped Ag into Zr-BEA catalyst and performed MES experiments, the most significant findings for Ag-Zr-BEA (Figure 4) can be found in the MS signals for COH and CA which were relatively small, suggesting that Ag-Zr-BEA has a different rate-determining step from Zr-BEA. In other words, AA does not need to be co-fed in Ag-Zr-BEA catalyzed ethanol coupling reaction. Also, the increasing signal at 2900-3000 cm-1 corresponds to C-H stretching of physisorbed EtOH on the surface. Although a small shoulder at 2900 cm-1 could be AA or CA species, there is no obvious C=O stretching at 1700 cm-1 to assign CA and AA due to Greenler surface selection rule.
Figure 4: Modulation experiment between EtOH under helium flow and pure helium flow over Ag-Zr-BEA at 320oC. (a) Recorded MS spectra and (B) diffuse reflectance FT-IR spectra.
The addition of Ag metal changes the ethanol coupling reaction such that it occurs in a single step; however, we still needed to elucidate the role of Ag metal. We synthesized a control catalyst, Ag-SiO2, which lacked Zr Lewis acid sites, to study the effect of Ag metal in the ethanol coupling reaction. The MS signal clearly showed that only AA is being produced and the signal for CA, COH, and BD appeared as flat lines (Figure 5), indicating that as hypothesized, Ag would function to dehydrogenate EtOH to AA. Additionally, there is no significant signal in the C=O stretching region, which indicates the fast desorption of AA on the surface. The C-H stretching region at 2900-3000 cm-1 shows the same features which corresponds to physisorbed EtOH on Ag-Zr-BEA. Therefore, the Ag metal can only dehydrogenate the EtOH in this control experiment.
Figure 5: Modulation experiment using EtOH under helium flow and pure helium flow over Ag-SiO2 at 320oC. (a) Recorded MS spectra and (B) diffuse reflectance FT-IR spectra.
From our results, we found that the one-step ethanol coupling reaction over Ag-Zr-BEA involves the Lewis acidity of Zr to perform coupling reactions of EtOH/AA mixtures (Aldol, MPV) coupled with the dehydrogenation functionality of Ag to form AA from EtOH. This grant has been used to support one graduate student who constructed the setup and performed the MES FT-IR experiments. During that period, one review paper regarding MES techniques and one research paper, described herein, were published. Also, the work was presented at an ACS meeting and the travel expenses were paid for by this grant.