Reports: ND956495-ND9: Understanding Local Dynamic Responses of Fixed Bed Reactors by CFD
Anthony G. Dixon, Worcester Polytechnic Institute
Coupling microkinetics with unsteady-state CFD has been studied to develop a method for tracking the dynamic behavior of surface sites in a fixed bed reactor. Initial transient simulations have been made for ethylene epoxidation in a small bed, and these show the start of thermal wave progression down the tube, and a maximum temperature above that given by steady-state models. Further developments will be used to investigate catalyst behavior under wrong-way and deactivating conditions.
Dynamic behavior of fixed bed reactors under transient conditions could lead to phenomena such as wrong way behavior and catalyst deactivation. These are highly unfavorable, that yet need to be addressed in the field. In order to study these phenomena a CFD integrated multiscale model is developed to study fixed-bed reactors under dynamic reaction conditions. In the proposed model a detailed reaction mechanism is coupled into the transport and flow in both the solid and fluid phases. The kinetics model is initially solved for a wide range of operating conditions under steady state and mapped into multivariate splines. Furthermore, generic computational libraries are constructed based on the splines. The libraries are imported into the CFD code to evaluate the reaction rates and surface species coverages. Since the surface species reach steady state much faster than temperature and concentration in the reactor it is a common practice to assume them to be at steady state even for transient simulations. Therefore, using the libraries for transient simulation is effective.
Ethylene oxidation over silver catalyst is studied using the developed model. The computational domain is an illustrative geometry of 25 spherical particles of size 4 mm in a tube of diameter 10.8 mm. Laminar flow is simulated, with inlet temperature and wall temperature of 490 K. The fluid and particles in the tube are initially at 478 K. A small time step (Dt = 0.25x/v where x is the mesh size and v is velocity) is used until the flow field reached steady state.
Figure 1 shows the evolution of the temperature profile with time. The flow direction is from right to left. It can be seen that it takes almost 6 s for the reaction to start and another 24 s for the catalytic bed to become fully reactive. Once all the particles are reactive the temperature rapidly increases which causes a heat wave along the reactor. The simulation shows that temperature during the reactor start-up rises significantly which could lead to an early deactivation of the particles.
Figure 1. Contours of temperature (K) profile evolution along the tube. Flow is right to left.
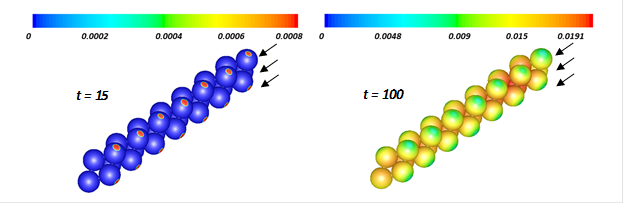
Figure 2 represents the reaction rate (kg/m3s) at t = 15 and t = 100 s. At t = 15 s the reaction is maximum on the surface of the catalyst near the tube wall and inlet because the temperature in those regions is higher than the initial temperature of the reactor. However, as the reaction becomes significant throughout the catalytic bed, the high temperature moves from the tube wall toward center of the tube where the reaction also becomes maximum. In other words, the tube wall now acts as coolant as it should and the reaction near that area has the lowest rate.
Figure 2. Contours of reaction rate (kg/m3s) on the surface of the catalyst particles during the transient operating time.
The results show the importance of the temperature profile evolution under dynamic reaction conditions and how it affects the reaction rate locally in the reactor. Using the developed method we will study effects of the wrong way behavior and moving hot spot on local reactivity and selectivity in fixed beds.
The research has allowed the PI to broaden his CFD capabilities into dynamic reactor phenomena, thus enabling study of a broader range of reactor performance and possible safety issues (e.g. moving hot-spots) than previously. The PhD student Behnam Partopour has been able to focus on research for the past year of his graduate study, thanks to support from this grant. The upcoming year he will also be supported as a research assistant on this grant, with an expected graduation date of May, 2018.