Reports: ND1056838-ND10: Endotaxially Anchoring Metal/Alloy Nanoparticles for Methanol Synthesis and Steam Reforming
Jingyue Liu, PhD, Arizona State University
To accomplish the proposed goals we first established a robust synthesis protocol for scaling up the production of high-quality ZnO NWs (Figure 1) which are used to support Pd, Au and Cu clusters and NPs. Our ZnO NWs are individually separable and can be dispersed easily in water, methanol or ethanol. The ZnO NWs consist of primarily {10-10} facets and are atomically flat as clearly revealed in the aberration-corrected scanning transmission electron microscopy (ac-STEM) image of Figure 1e. The diameters of the ZnO NWs range from ~10 nm to 100 nm with an aspect ratio of ~ 100. Since the ZnO NWs are enclosed by the lowest energy surfaces they are extremely stable at calcination temperatures < 700°C.
Figure 1. Synthesis of ZnO NWs as catalyst support materials (a and b), SEM image of the synthesized ZnO NWs (c), high-angle annular dark-filed (HAADF) STEM image of the ZnO NWs (d), and atomic resolution HAADF image of a ZnO NW showing the flat {10-10} nanoscale facet.
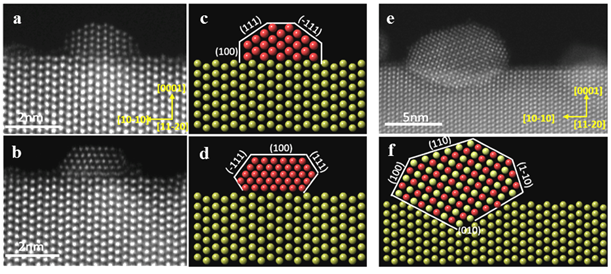
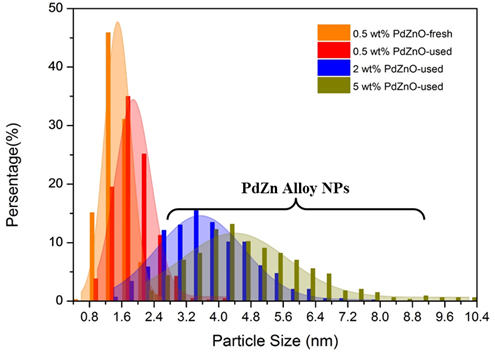
With such readily available high-quality ZnO NWs, we systematically synthesized ZnO NW supported Pd catalysts with various amounts of Pd loading. These Pd/ZnO precursor materials were reduced at various temperatures under forming gas environment. We discovered that with low level loadings of Pd (< 0.2wt%) the Pd existed as single atoms. With increasing levels of Pd loading, Pd clusters (< 1 nm) and faceted small Pd NPs (< 2 nm) formed and these Pd clusters and small Pd NPs persisted even under forming gas reduction at temperatures up to 300°C (Figure 2a and 2b). The facets of the small Pd NPs and their epitaxial relationship with the ZnO NWs were schematically illustrated in Figure 2c and 2d. With high levels of Pd loading (> 1.0wt%) and under forming gas reduction at temperatures > 250°C, the Pd NPs, however, were transformed into PdZn alloy NPs (Figure 2e). Furthermore, the PdZn alloy NPs are partially embedded into the {10-10} nanoscale facets of the ZnO NWs to establish an endotaxial relationship (Figure 2f). Detailed particle size analyses were conducted on numerous ac-STEM images of the various Pd/ZnO and PdZn/ZnO catalysts and the size distributions were plotted for different Pd loading levels (Figure 3).
Figure 2. Atomic resolution HAADF images of Pd NPs epitaxially grown on ZnO NWs (a, b) and PdZn alloy NPs endotaxially grown into ZnO NWs (e). The corresponding schematic illustrations were shown in c, d and f, respectively.
Figure 3. Size distributions of Pd and PdZn NPs in the Pd/ZnO NW and PdZn/ZnO NW catalysts with various loading levels of Pd. The size distribution of the Pd NPs in the 0.5wt%Pd/ZnO catalyst after MSR reaction is also shown for comparison. Small Pd clusters grew bigger after the MSR reaction.
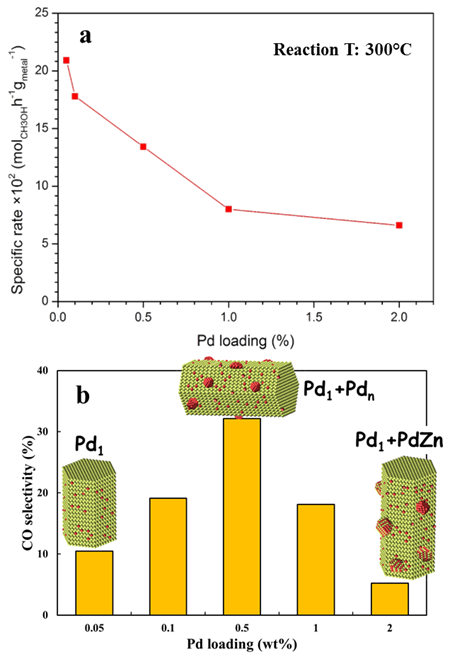
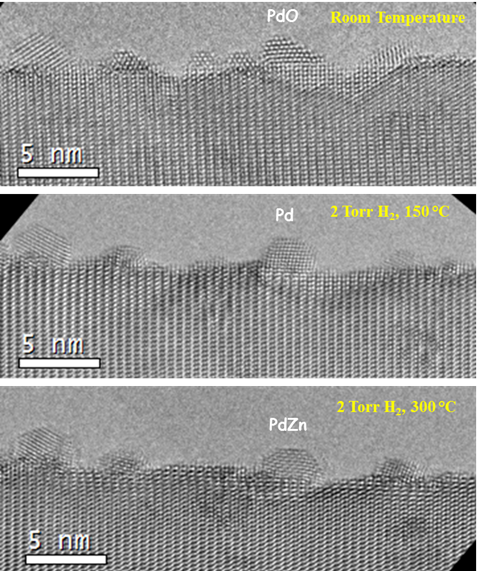
We tested the catalytic performance of the synthesized Pd/ZnO and PdZn/ZnO catalysts for the MSR reaction. The specific activity for methanol conversion decreases with the Pd loading (Figure 4a). The Pd1/ZnO single-atom catalyst possesses the highest specific activity since in this case each Pd atom acted as an active center. The selectivity toward CO on these catalysts shows a more interesting behavior (Figure 4b). During the MSR reaction, both the ZnO supported single Pd atoms and the PdZn alloy NPs provided a low selectivity toward CO (i.e., high selectivity toward CO2). The ZnO supported Pd clusters or small NPs, however, favored the production of high amount of CO, resulting in highest CO selectivity on the catalyst with 0.5wt% Pd loading. Based on the ac-STEM images, the distribution of the Pd on the ZnO NWs with various levels of Pd loading is schematically illustrated in the insets of Figure 4b. The small Pd clusters grew in size during the MSR reaction as shown in Figure 3. Our results suggest that small Pd clusters do not form PdZn alloys even after reduction treatment and these ZnO supported Pd clusters yield high CO selectivity. We also conducted in situ investigation of the dynamic evolution of the Pd/ZnO precursors during the reduction process (Figure 5).
Figure 4. Specific activity for MSR reaction over Pd/ZnO catalysts with different Pd loading levels (a) and selectivity toward CO (b).
Figure 5. Aberration-corrected environmental TEM study of the dynamic evolution of the Pd/ZnO NW precursor material during H2 reduction process. The PdO NPs were reduced to Pd NPs at 150°C under 2 Torr H2 and tt 300°C PdZn alloy NPs were formed.
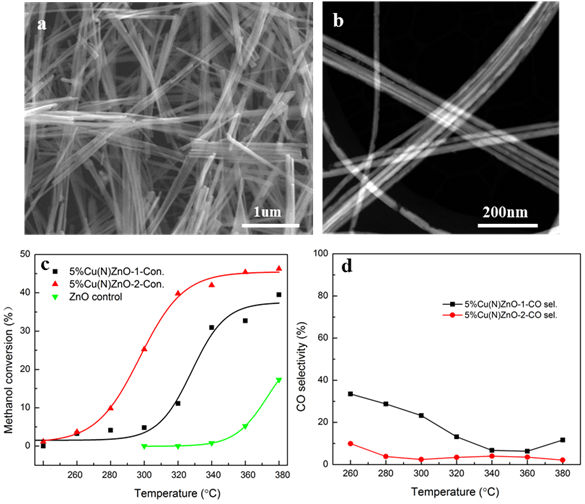
During this reporting period, we have also synthesized Cu NWs and explored the catalytic behavior of mixing Cu and ZnO NWs for MSR reaction. The Cu NWs possess a size and aspect ratio similar to those of the ZnO NWs (Figure 6a and 6b). The methanol conversion rate and the CO selectivity are displayed in Figure 6c and 6d, respectively. The performance of this catalyst became better (higher methanol conversion and lower CO selectivity) as the catalyst was used. From these data we concluded that the intimate contact between the Cu and ZnO NWs may play a major role in determining both the catalytic activity and selectivity of the mixed Cu and ZnO NW catalyst. We are in the process of verifying this proposed mechanism and synthesizing ZnO NW supported Cu and Au NPs.
Figure 6. SEM (a) and ac-STEM (b) images of Cu NWs, methanol conversion (c) and CO selectivity (d) for MSR reaction on a catalyst consisting of Cu and ZnO NWs. The increase of methanol conversion and the decrease of CO selectivity for the 2nd cycle run are presumably due to the enhanced amount of intimate contacts between the Cu and ZnO NWs.