Reports: UR153693-UR1: Intramolecular Cycloaddition Reactions of Electron-Rich Alkynes: New Methods for the Synthesis of Optically Active Building Blocks for Organic Synthesis
Thomas G. Minehan, California State University, Northridge
Year 3 ACS-PRF Narrative Progress Report: Thomas Minehan
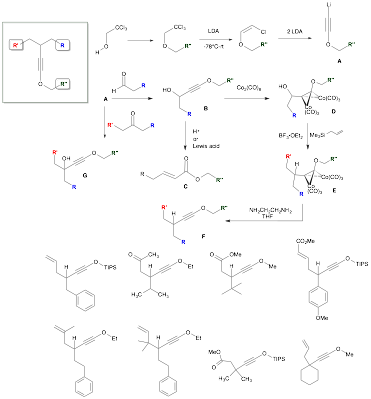
Our investigation of the chemistry of electron-rich alkynes (ynol ethers) over the past year has led to the discovery of new methods for the preparation of complex substituted ynol ethers. Trichloroethanol may be easily alkylated under basic conditions and then treated exhaustively with LDA to effect dehydrohalogenation and provide lithioalkynyl ether A (Scheme 1).1 Reaction of A with aldehydes and ketones then furnishes propargylic alcohols B and G in high overall yields. However, further functionalization of the propargylic position of B is hampered by the well-known Meyer-Schuster rearrangement,2 which takes place under Brønsted or Lewis-acidic conditions to provide enoate C. To avoid this, the ynol ether may be protected as its cobalt complex D, which then easily undergoes the Nicholas reaction3 with diverse nucleophiles (allyl silanes, silyl enol ethers, silyl ketene acetals, and electron-rich aromatics) under Lewis acidic conditions to provide E. Decomplexation is easily effected under mild basic conditions4 to afford a variety of complex ynol ethers F, containing diverse substituents R, RI and RII.
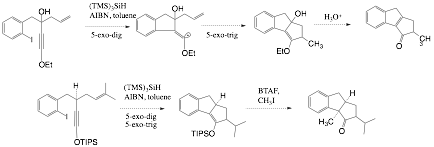
With this method in hand, we are currently exploring tandem intramolecular radical addition reactions of alkene- and halide-tethered alkynyl ethers (Scheme 2). The addition of a carbon-centered radical to an ynol ether is expected to occur in a regioselective fashion to generate an alpha-oxo alkenyl radical,5 which can subsequently combine with an olefin to generate a product containing two new rings.
The product also generates an enol ether regioselectively, which can subsequently be engaged in reactions with electrophiles to generate an additional stereogenic center. Silyl enol ethers, obtained by tandem radical reactions from silyl ynol ethers, are most interesting in this regard, as the enolate form can be generated under mild conditions with benzyl trimethylammonium fluoride (BTAF).6
Scheme 2. Exploring the radical reactions of ynol ethers
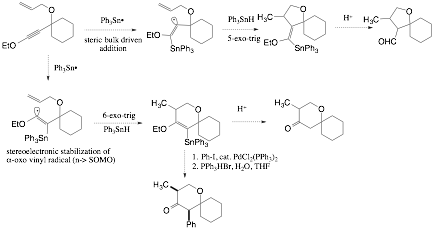
Tandem inter- and intramolecular radical reactions of ynol ethers are also an important area of current interest in our research program. Intermolecular and intramolecular addition of carbon radicals to ynol ethers has been shown by Daoust5,7 to proceed in a regioselective manner to produce alpha-oxo vinyl radicals. We are currently exploring the addition of triphenyltin radical to ynol ethers containing tethered alkenes as radical acceptors. We are interested in assessing if steric bulk or stereoelectronics controls the site of tin radical addition to ynol ethers; subsequent 5-exo-trig or 6-exo-trig cyclization of the alpha-oxo vinyl radical would then produce substituted 5- or 6-membered ring systems, indicating the regioselective preference for the intermolecular process. It has also been shown by Hale8 that propargylic alcohols and ethers direct addition of triphenyltin radical to the proximal carbon of the triple bond of alkynes. We are investigating this heteroatom effect with ynol ethers bearing propargylic hydroxyl groups and ethers bearing pendant alkene tethers capable of engaging the alpha-oxo radical in intramolecular 5-exo-trig cyclization reaction. Tin radicals will be replaced with carbon-centered radicals once the conditions for regioselective additions to the ynol ether have been elucidated. The products of these processes are highly substituted cyclopentanoid rings that are scarcely accessible by other synthetic methods.
Scheme 3. Regioselectivity of tandem inter- and intramolecular radical reactions of ynol ethers.
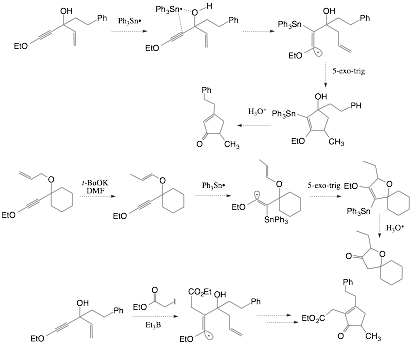 |
Scheme 4. Directed addition of stannanes to ynol ethers.
References.
- Pirrung, M.C.; Hwu, J. R. Carbene Chemistry. Stereoselective Synthesis of Haloalkenes. Tetrahedron Lett. 1983, 24, 565-568.
- Meyer, K.H.; Schuster, K. Umlagerung Tertiarer Athinyl-carbinole in Ungesattigte Ketone. Berichte 1922, 55, 819.
- Nicholas, K. M. Chemistry and Synthetic Utility of Cobalt-Complexed Propargyl Cations. Acc. Chem. Res. 1987, 20, 207-214.
- Sugihara, T.; Ban, H.; Yamaguchi, M. Novel Decomplexation Method for Alkyn-Co2(CO)6 Complexes. J. Organomet. Chem. 1998, 554, 163-166
- Longpre, F.; Rusu, N.; Larouche, M.; Hanna, R.; Daoust, B. Radical Addition to Ynol ethers – A Convenient Procedure for the Stereoselective Preparation of alpha-Iodo Enol Ethers in Neutral Conditions. Can. J. Chem. 2008, 86, 970-975.
- Kuwajima, I.; Nakamura, E.; Shimizu, M. Fluoride-Mediated Reactions of Enol silyl ethers. Regiospecific Monoalkylation of Ketones. J. Am. Chem. Soc. 1982, 104, 1025-1030.
- Hanna, R.; Daoust, B. Intramolecular Regioselective Addition of Radicals and Carbanions to Ynol Ethers. A Strategy for the Synthesis of Exocyclic Enol Ethers. Tetrahedron 2011, 67, 92-99.
- Dimopoulos, P.; Athlan, A.; Manaviazar, S. George, J.; Walters, M.; Lazarides, L.; Aliev, A. E.; Hale, K. J. O-Directed Free-Radical Hydrostannations of Propargyl Ethers, Acetals, and Alcohols with Ph3SnH and Et3B. Org. Lett. 2005, 7 5369-5372.