Reports: ND753739-ND7: Nanostructured and Nanoporous Composites from Nanoparticle Jamming during Polymerization Induced Phase Separation
Bryan D. Vogt, University of Akron
The primary objective associated with this project is to use nanoparticles during polymerization induced phase separation to control and modify the morphology to improve the properties of the nanocomposite blends. The initial system used poly(hydroxyethylmethacrylate), titania nanoparticles, and furfuryl alcohol. A photoacid was used to catalyze the polymerization of the furfuryl alcohol, but the nanostructure was not significantly altered by the selection of conditions. This lack of structural control with small nanoparticles led us to examine 2D materials as the nanoparticle additive. We selected graphene oxide (GO) as the 2D material, but when this was mixed with branched poly(ethylenimine) (BPEI) the GO flocculated.
As phase separation occurs, we initially focused on characterizing the properties of these aqueous mixtures of BPEI and GO as described last year.
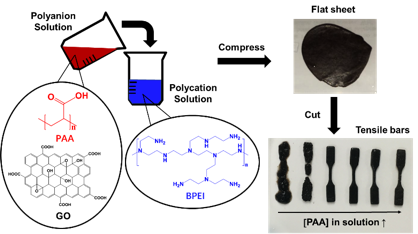
From these investigations, we found that hydrogel materials of GO and BPEI could be tuned for their mechanical properties by changing the ratio of GO and BPEI in the mixture. However, one challenge was that these GO-BPEI hydrogels were extremely brittle. We hypothesized that the inclusion of a flexible polyanion could provide a route to toughen these materials. Fig 1 schematically illustrates how the material is fabricated from aqueous assembly.
Fig 1. Schematic illustrating the protocol to fabricate the GO/PAA/BPEI complex and to prepare the samples for tensile tests.
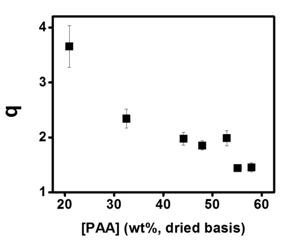
The equilibrium mass swelling ratio (q) (defined as the mass of swollen material divided by the mass of dried material and shown in Fig. 2) provides insight into how the PAA changes the charge compensation of the complex. Considering that the amount of positively charged BPEI in the complex remains at the same level, the increasing [PAA] means that a higher fraction of the amine groups on BPEI are occupied by PAA. The balancing of the charges within the complex by matching the charge densities of weak polycations and polyanions leads to less free charges that contributed to decreased hydration of the complex. As the local density of charge groups on the BPEI and PAA are similar, the PAA can better compensate the charges on the BPEI chain as opposed to the GO where the charges are more distributed.
Fig 2. Equilibrium mass swelling ratio (q) of GO/PAA/BPEI complex as a function of the PAA concentration in the dried complex
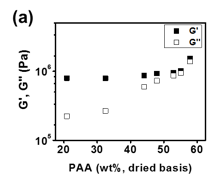
Variation in rheological behaviour of complexes can provide insight into the microstructure of the complex as a function of composition. Fig. 3a shows how G’ and G’’ of the complexes at 1 rad/s are impacted by [PAA]. Both G’ and G’’ increase with increasing [PAA]. This can be interpreted as the effect of the change in both crosslink density and hydration extent. Due to increased flexibility of PAA chains compared to rigid GO as well as increased density of negative charges along the PAA backbones compared that of GO, PAA can form a larger density of ion pairs with BPEI than the GO can. This results in a decrease in equilibrium mass swelling ratio and an increase in crosslink density simultaneously. The increased crosslink density leads to a mechanically stronger network structure.
Fig 3. At ω = 1 rad/s, (a) storage modulus G’(■) and loss modulus G’’(□), (b) tan δ of GO/PAA/BPEI complex with q = 1.45 ~ 3.66
A higher crosslink density should also increase the elastic response of the materials. However, as shown in Fig 3a., the difference between G’ and G’’ decreases as [PAA] increases and the complex become less hydrated. The increase in G’’ as [PAA] increases is associated with the ability of the complex to dissipate energy. The intrinsic rigidity of the GO appears to limit the viscous losses while the addition of the PAA would be expected to increase the viscous losses. This effect can be seen from Fig 4b, which shows the loss factor (tan δ) of the complex as a function of [PAA] indicating the viscoelastic behaviour of the GO/PAA/BPEI was changing from solid-like towards liquid-like as [PAA] increased.
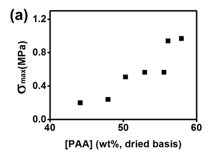
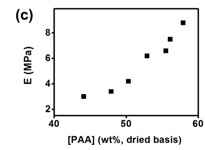
Fig 4 illustrates the tensile properties obtained from the stress-strain curves as a function of [PAA]: tensile strength (smax), elastic modulus (E), strain at failure (emax) and toughness (UT). Generally, as [PAA] increases, smax and E both increase as a result of the increasing crosslink density and decreasing swelling ratio; while UT and emax first increase with increasing [PAA] until a maximum is reached at [PAA] = 52.9 wt%. Not all the compositions were able to be tensile tested. Only when [PAA] = 44.1 ~ 57.9 wt % ([PAA]sol = 120 ~ 280 Mm) in the materials can the tensile tests be performed due to the brittle nature of the complexes containing high GO content.
Fig 4. Tensile properties (a) tensile strength (smax); (b) failure strain (emax); (c) Young’s modulus (E) (d) failure energy (UT); of the GO/PAA/BPEI complexes
The variation in mechanical properties can be interpreted from consideration of the thermodynamics and kinetics of PEC formation. Having dimensions on the order of several microns, the 2D GO sheets are much larger in size than BPEI or PAA chains. Due to hindered size, shape, and flexibility, it is more difficult for GO sheets to participate in the exchange process. The GO sheets in the system may locally hinder polymer diffusion. We postulate that when the two oppositely charged polyelectrolytes are interacting a greater degree of crosslinking is achieved compared to the case of one polyelectrolyte and the large GO sheets.
Although this ultimate direction of the project was focusing on interactions rather than polymerization to control the structure and properties of nanocomposites, this still represented a new area of research for my group. We have several additional manuscripts in preparation regarding these materials. This work has provided a new direction in research that will be continued to be pursued over the next several years. One MS student graduated and is now a PhD student at Drexel University. The original Ph.D. student on this project also successfully graduated.