Reports: DNI556249-DNI5: Probing the Ionic Liquid-Vapor Interface by Ambient Pressure X-ray Photoelectron Spectroscopy
John Newberg, PhD, University of Delaware
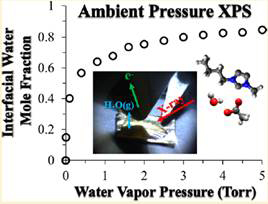
There has been a tremendous amount of effort in the literature to understand ionic liquid (IL) interfaces using vacuum-based X-ray photoelectron spectroscopy. While this method continues to increase our fundamental understanding of the IL-vacuum interface, little is known about ILs under ambient conditions using photoemission techniques. This is due, in part, to the paucity of ambient pressure photoemission instruments available, and the technological difficulties of performing such experiments. The focus of our work has been utilizing a recently commissioned ambient pressure X-ray photoelectron spectroscopy (APXPS) instrument in our laboratory to probe the IL-gas interface under Torr level gas phase pressures. Recently, we reported the first pressure-dependent APXPS study of an IL interface by examining 1-butyl-3-methylimidazolium acetate, [BMIM][OAc], in the presence of water vapor (Figure 1). [BMIM][OAc] is a hydrophilic IL that is known to absorb water into the bulk as evidenced by gravimetric and IR studies in the literature. Our APXPS studies showed that water is also significantly present at the IL-gas interface. A number of fundamental findings resulted from our APXPS work, including: i) water in the interfacial region rapidly equilibrates with the surrounding humidity while bulk absorption is much slower, ii) the first pressure-dependent, quantitative assessment of the interfacial region shows a monotonic rise in interfacial water mole fraction with increasing pressure, with a maximum of 0.85 at 5 Torr water vapor at room temperature, iii) increasing water in the interfacial region leads to significant changes in the electronic environment of both the acetate anion and the imidazolium nitrogen in the cation, and iv) at 0.7 water mole fraction there is a significant change in the IL interfacial structure attributed to an onset in nanostructuring driven by the separation of the water/IL mixture into hydrophilic and hydrophobic regions. These results suggest that one can control interfacial nanostructuring by merely changing the surrounding relative humidity. The results of these studies have spawned collaborations with theoretical chemists to perform DFT and MD simulations in order to elucidate the effects of water on the electronic environment and interfacial dynamics.
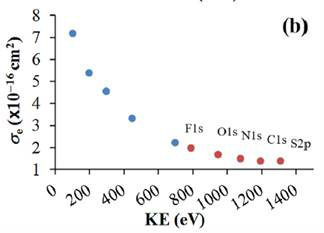
We have also examined the IL-gas interface using a hydrophobic IL, 1-ethyl-3-methylimidazolium-bis-(trifluoromethylsulfonyl)-imide, [EMIM][Tf2N]. APXPS experiments up to a maximum of 2 Torr water vapor pressure at room temperature showed no evidence of water uptake in the interfacial region. Taking advantage of this hydrophobicity, we performed the first kinetic-energy dependent electron scattering cross section studies using lab-based APXPS. X-ray photoemission studies historically have required the use of a synchrotron to vary the X-ray energy in order to study the electron scattering cross sections as a function of kinetic energy. We have taken advantage of the chemical diversity of [EMIM][Tf2N] to examine the electron scattering cross section of water vapor over a wide range of 520 eV KE. The lab-based APXPS results nicely complement synchrotron-based APXPS studies (Figure 2). These results highlight the powerful utility of lab-based APXPS to determine electron scattering cross sections as a function of KE without the need for a variable X-ray source.
Figure 1. APXPS investigation of hydrophilic [BMIM][OAc] in the presence of water vapor.
Figure 2. APXPS investigation of hydrophobic [EMIM][Tf2N], showing the electron scattering cross section of water vapor as a function electron kinetic energy. Blue data are from synchrotron-based APXPS studies, while red data are from our lab-based APXPS results.