Reports: ND253800-ND2: The Compound-Specific Sulfur Isotopic Signature of Dissolved Organic Matter Sulfurization
Josef P. Werne, PhD, University of Pittsburgh
Understanding how and why organic sulfur forms in oils and source rocks is a key question, and the application of compound-specific S isotope analysis (CSSIA) to such questions is likely to be informative. Preliminary CSSIA data indicate that the sulfur isotope composition (d34S) of specific organic sulfur compounds (OSC) vary substantially (Raven et al., 2015). Thus, OSCs must derive from multiple pools of inorganic sulfur with d34S values that vary in time and space. Furthermore, different reaction pathways involving different substrates will impart additional fractionations, leading to a substantial range of d34S in individual compounds. This study is designed to (1) determine the sulfur isotope fractionation associated with the sulfurization of organic matter and (2) investigate an organic rich sedimentary system to determine whether the sulfur isotope signals expected are preserved in geological samples or overprinted during diagenesis.
Progress to date
Polysulfide analysis
Polysulfides play a significant role in environmentally relevant processes because of their redox reactivity and nucleophilic tendencies, but determining polysulfide speciation in the natural environment is challenging. We are developing methods for the direct measurement of d34S of each polysulfide species (S22- to S82-) at concentrations present in natural samples using a GC coupled to a multicollector ICP-MS (GC/MC-ICP-MS).
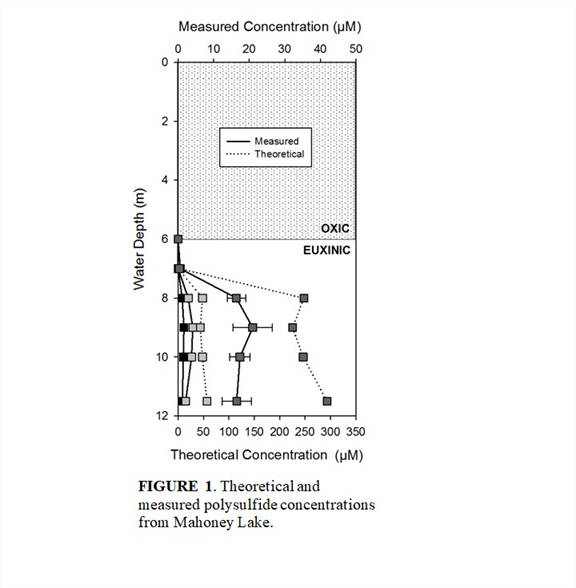
Our refined method currently allows for the complete separation and detection of S22- to S52-, but can be further optimized for lower detection limits and better recoveries. Repeated measurements over a two week period showed that derivatized samples maintain 80% of their original concentration when stored at 2°C. However, once prepared for analysis, the polysulfides quickly degrade, so immediate analysis is required once final preparation is complete. Distributions of dimethylpolysulfanes from the Mahoney Lake, British Columbia water column match theoretical calculations, but the absolute concentrations are offset by an order of magnitude (Figure 1) . The reasons for this offset are most likely explained by (a) inefficient field derivatization, (b) time until extraction and analysis, and/or (c) polysulfides reacting in the water column so their abundance is lower than expected.
d34S of laboratory-sulfurized compounds
Previous laboratory experiments have suggested that the S isotope fractionation during the formation of OSCs depends on the mechanism of S incorporation and the original functionality of the organic molecule (Amrani et al. 2008). Compound-specific studies of the Cariaco Basin show large variations in the δ34S values of individual OSCs (Raven et al. 2015, Werne et al. 2008). Thus, a key question remains, “What is (are) the sulfur isotope fractionation(s) associated with the sulfurization of organic matter?”
We are analyzing several organic compounds, including carbohydrates (glucose), lipids (5α-Cholestan-3-one), and natural dissolved organic matter (DOM) sulfurized under conditions representing those in the natural environment. Sulfurized reaction products and associated inorganic sulfur species have been identified, and bulk d34S measurements of reactants and products have been made.
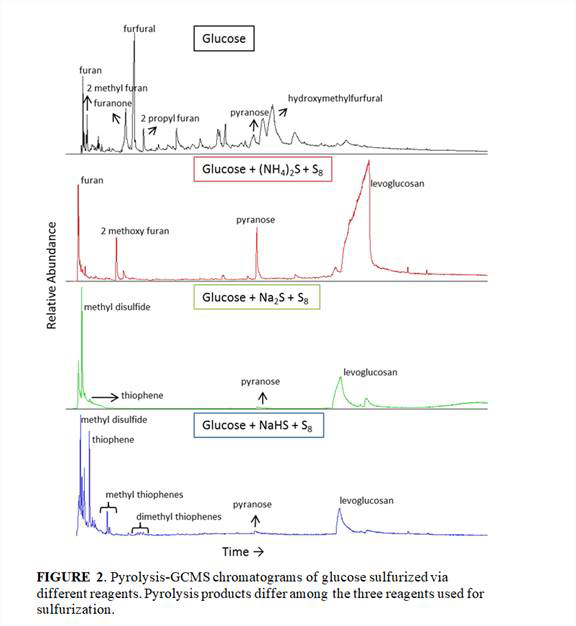
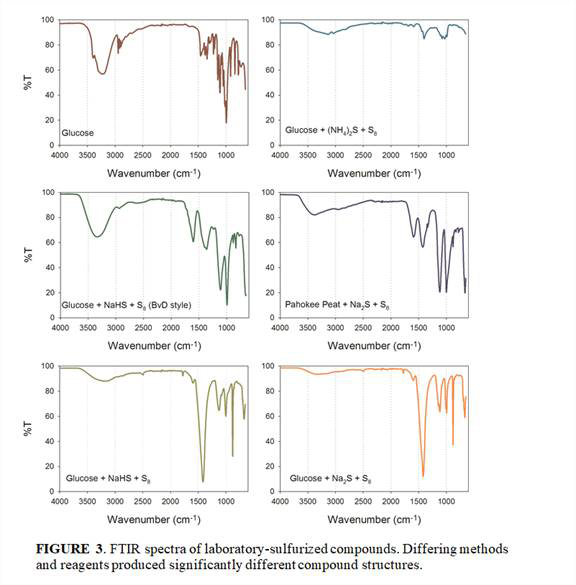
Online pyrolysis-GCMS and FTIR analysis of the extracted residues of our glucose and DOM sulfurization experiments indicated that the specific methods and reagents used to produce polysulfide solutions resulted in significantly different organic sulfur compounds, which was not previously recognized (Figures 2 and 3).
Compound-specific d34S of OSC from the Kimmeridge Clay Formation, UK
The Blackstone Band of the Jurassic Kimmeridge Clay Formation (KCF) has an organic carbon content of more than 50%, which has been attributed to the selective preservation of carbohydrates via sulfur incorporation in the euxinic water column (van Dongen et al. 2006).Comparison of structure and d34S of KCF OSC to the laboratory-sulfurized compounds will be used to confirm whether the organic enrichment is caused by sulfurization of carbohydrates in the marine water column. -
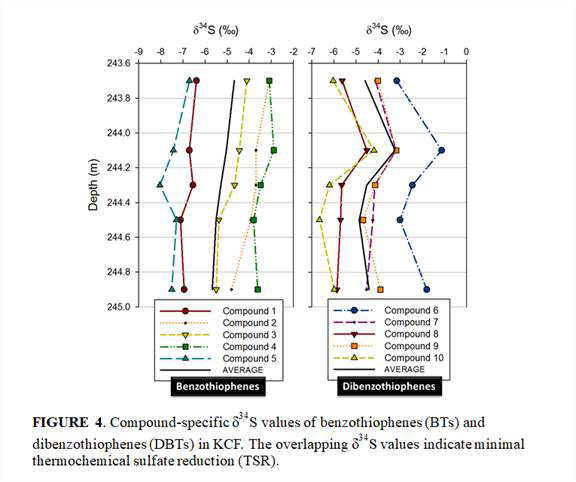
Initial δ34S measurements of lipids, benzothiophenes (BTs) and dibenzothiophenes (DBTs), indicated that the samples have not undergone significant thermochemical sulfate reduction (Figure 4).
Offline pyrolysis of KCF residues also produced BTs and DBTs within the pyrolysates. We will measure their δ34S when the instrument at Caltech is available (it is currently undergoing an upgrade), and compare it to the extractable BTs and DBTs δ34S values.
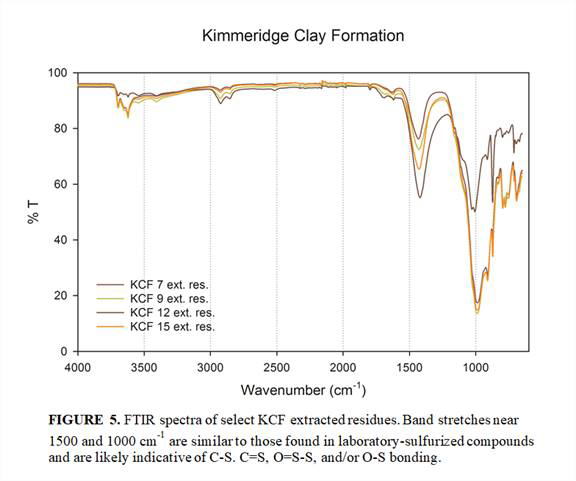
FTIR analysis of select KCF residues was completed (Figure 5) and comparison of spectra with those of the laboratory-sulfurized compounds revealed structural similarites. The FTIR similarities potentially provide a quick and easy diagnostic tool of sulfurization conditions in natural samples.
Timeline to completion
Sulfurization reactions, identification and quantification of extractable OSC, and inorganic S measurements were completed as of Fall 2016. Pyrolysis-GCMS and FTIR was completed in January 2017 at the University of Manchester with collaborator Dr. Bart van Dongen. Compound-specific δ34S measurements will be completed in 2017 at Caltech with collaborator Dr. Alex Sessions, once the MC-ICP-MS instrument is upgraded and working properly).
Impact of research
The research supported by this ACS-PRF grant represents a significant step forward in our understanding of the sulfurization of organic matter in natural systems. The development of improved methods will facilitate the first ever sulfur isotope analyses of individual polysulfides, which is a key factor in understanding this process. The support provided to two graduate students has allowed them to focus full time on their research into organic and stable isotope biogeochemistry, and forms the core of one students PhD dissertation, from which I anticipate 2-3 publications related to PRF supported research will be produced in the coming year.
References
Amrani A. et al. (2005) Org. Geochem. 36:971-74
Amrani A. et al. (2008) Chem. Commun. 2008:1356-58
Amrani, A. et al. (2009) Anal. Chem. 81:9027-9034.
Raven, M. et al. (2015) Org. Geochem. 80:53-59.
van Dongen, B. et al. (2006) Org. Geochem. 37:1052-1073.
Werne, J. et al. (2008) Geochim. Cosmochim. Acta 72:3489-3502.