Reports: DNI555373-DNI5: In Situ NMR Studies of the Hydrogen Spillover Phenomenon in Heterogeneous Catalysis
Yan-Yan Hu, PhD, Florida State University
Introduction
The objective of our research is to understand in sufficient detail how activated H atoms migrate from the surface of a catalyst to the surface of the catalyst support, i.e., hydrogen spillover (Fig. 1A). To accomplish this goal, solid-state NMR is employed to study hydrogen migration and participation in the reactions catalyzed by catalyst-metal oxide support systems, including Pt/SiO2, Pt/Al2O3, and Pt/TiO2.
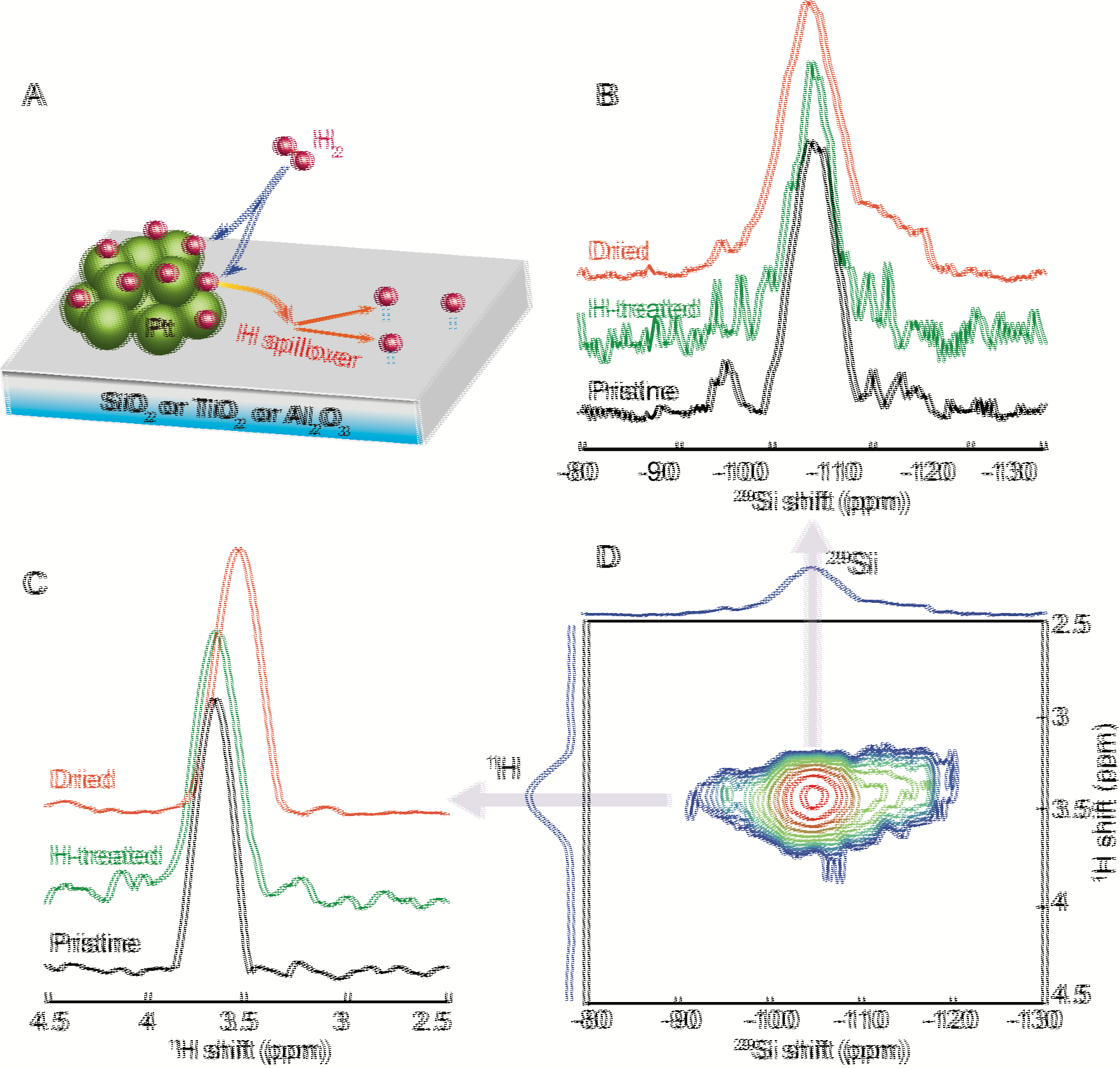
Figure 1 A. Schematic representation of H spillover from Pt to metal oxide substrate surface, B. 29Si projection spectra and C. 1H projection spectra extracted from D. 2D 1H-29Si HETCOR spectra of pristine, H2(g) treated, and dried Pt-SiO2.
Current Progress
The catalyst-metal oxide support composites, i.e., Pt/SiO2, Pt/Al2O3, and Pt/TiO2 were prepared following a reported method1.
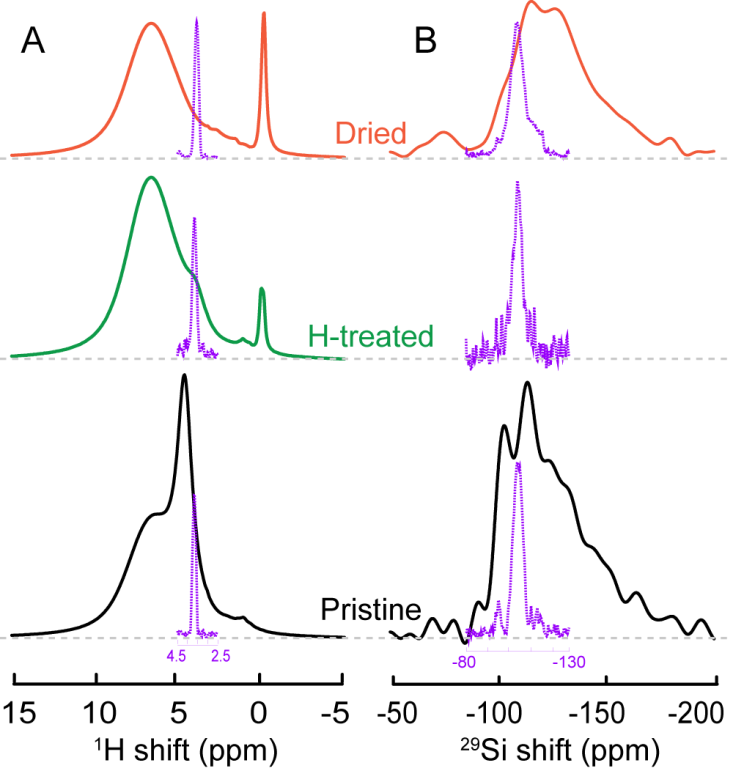
Multinuclear multi-dimensional NMR experiments have been carried out to examine the surface structure of metal oxide catalyst support and Pt catalyst. Pt/SiO2 was first examined as a model system. Since surface H and SiO2 are most relevant to catalysis, 1H-29Si Heteronuclear Correlation (HetCor) experiments with 1H homenuclear decoupling have been performed to obtain high-resolution 1H and 29Si NMR of the catalyst support surface (Fig. 1D). In two-dimensional 1H-29Si HetCor experiments, a peak at (dA, dB) is observed if a 1H species with a chemical shift dA correlates with a 29Si species with a chemical shift dB. As seen in Fig. 1D, a 1H resonance at 3.6 ppm correlates with a 29Si species resonating at -105 ppm. This 3.6-ppm resonance shifts to higher field (smaller ppm value) for Pt-SiO2 after drying at 200oC overnight. The comparison of the total 1H NMR spectra of Pt-SiO2 before and after H2(g) treatment (Fig. 2) reveals significant increase in the intensity and fraction of the 3.6-ppm resonance after H2(g) treatment. This 3.6-ppm resonance may be the active surface H that participates in catalytic reactions.
Figure 2 Comparison of 1D 1H and 29Si Hahn-echo spectra and the projection spectra from two-dimensional 1H-29Si HETCOR with the latter plotted in purple.
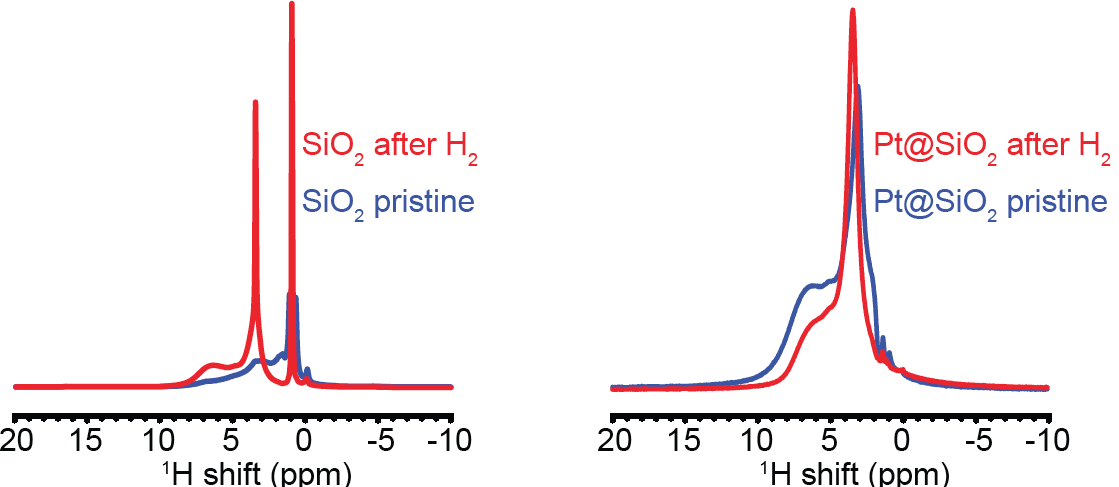
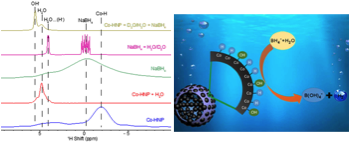
The selective 1H NMR spectra of SiO2 and Pt@SiO2 before and after H2(g) treatment are shown in Fig. 3. The intensity of the 3.6-ppm resonance in the 1H spectra of SiO2 increases significantly after H2 treatment. The narrow linewidth suggests high mobility of the 1H species resonating at 3.6 ppm, which is likely from loosely adsorbed H2 gas on the SiO2 surface. For Pt@SiO2 after H2(g) treatment, a 3.6-ppm broad resonance emerges. This is likely from the Pt-activated H spilled over and adsorbed on the SiO2 surface. Pt-activated H on the Pt-surface is expected to resonate at < 0 ppm. Due to the small amount of Pt (5 wt%), the Pt-H complex is not detected. Instead, a similar model system Co-activated H2 is used as a reference (Fig. 4). In the Co-catalyst system, H2 adsorbs onto Co surface and is activated to form H. The Co-H complex is detected in the 1H NMR spectrum at – 2 ppm2.
Figure 3 Comparison of 1H spectra from SiO2 and Pt-SiO2 before and after H2(g) treatment.
Figure 4 1H spectra from Co-catalyzed H2 formation reactions and the schematic illustrating the mechanism of Co-catalyzed H2 generation2.
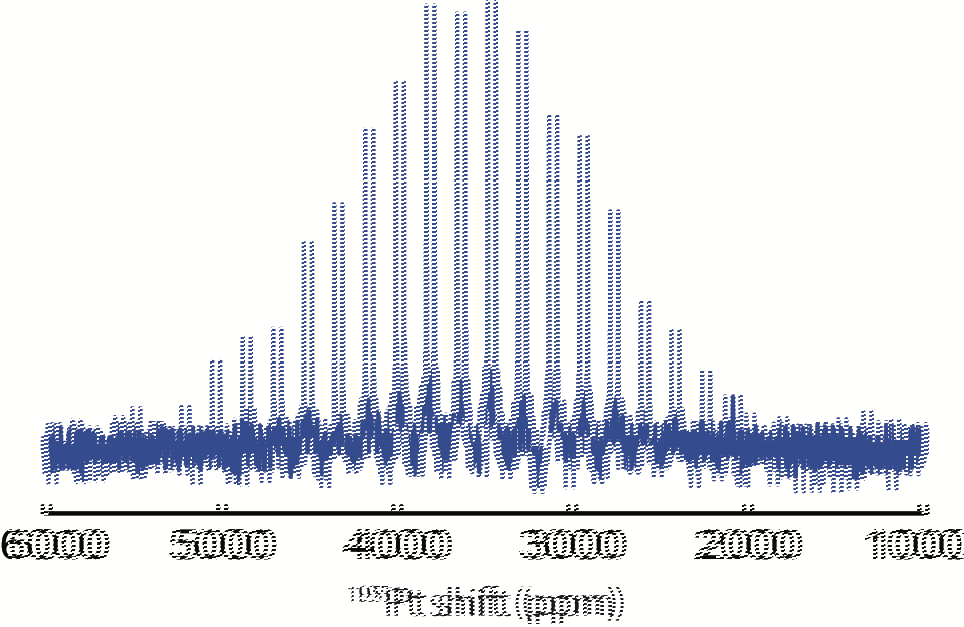
195Pt NMR was also acquired and shown in Fig. 5. The large disposition (> 2,000 ppm) of the peak from 0 is due to interactions of Pt nuclei with electrons at Fermi level, which leads to Knight shift, typical of metals. The broad peak with a width of ~ 4,000 ppm poses challenges to build 1H-195Pt correlations. However, recent studies have shown that with fast sample spinning and low 1H background, it is possible to achieve 1H-detected 1H-195Pt correlations, which will be carried out in our future work.
Fig 5 195Pt NMR spectrum acquired with a Carr-Purcell-Meiboom-Gill) CPMG pulse sequence.
Summary and Future Work
The possible spilled-over H species on SiO2 surface have been identified with high-resolution 1H-29Si correlation experiments, i.e. the 3.6-ppm resonance. This resonance grows significantly stronger in SiO2 and Pt-SiO2 after H2(g) treatment, indicating that the 3.6-ppm 1H resonance is associated with H2 adsorption and activation. This investigation will be further extended to the Pt-TiO2 and Pt-Al2O3 combinations to understand the variation of the H-spillover phenomena on substrates with different electronic conductivities. One paper has been published based on this work2 and another manuscript is being prepared for submission in the next 6 months.
Impact on Career and Participating Students
This award has provided the opportunity for an African American female student Ms. Alyssa Rose to participate in graduate research supervised by a senior postdoc Dr. Xuyong Feng and the PI. This project forms part of Ms. Rose's thesis on "in situ studies of energy-related materials". She has published a paper in Journal of Physical Chemistry C and is preparing another manuscript on this project. She gave a conference talk at the Florida Annual Meeting and Exposition (FAME) and has successfully defended her PhD candidacy based on what she has achieved in understanding the Hydrogen spillover phenomenon. This grant also allowed the PI's research group to establish unique research capabilities for studying surface/interface chemistry, which has facilitated the initiation of other research directions on interface chemistry within the PI's group. As a result, the PI has been granted funding from the AAAS to investigate interface chemistry of composite materials and selected for the Marion Milligan Mason Award in 2016. The support from ACS PRF has helped to build the foundation for achieving the PI's career goals, i.e., to establish a fundamental understanding of the correlation between interface chemistry and interfacial reactions and to nurture the next generation of young scientists.
References
1. S. H. Joo, J. Y. Park, C.-K. Tsung, Y. Yamada and P. Yang, "Thermally stable Pt/mesoporous Silica Core-Shell Nanocatalysts for High-temperature Reactions", Nat. Mater. 8 (2008) 126-131.
2. B. Liu, A. Rose, N. Zhang, Y.-Y. Hu, M.Ma, "Efficient Co-Nanocrystal-Based Catalyst for Hydrogen Generation from Borohydride", J. Phys. Chem. C 121 (2017), 12610-12616.