Reports: ND856166-ND8: Temperature-Dependent Physical Properties of Evaporites and Related Rocks: New Constraints on the Thermal Evolution of Sedimentary Basins
Alan G. Whittington, PhD, BA(Hons) , University of Missouri, Columbia
In year 1 of the grant, we have investigated the thermal properties of three main types of sedimentary rocks: evaporites, carbonates and shales. We have also tested thermal models to show how variations in thermal conductivity as a function of mineralogy, texture, and temperature affect geothermal gradients and maturation windows in sedimentary basins.
1. Shales.
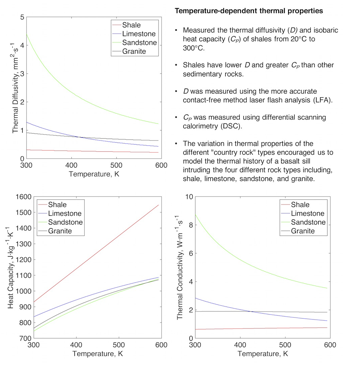
We measured the thermal diffusivity (D) and isobaric heat capacity (CP) of 8 different shales from 20°C to 300°C. Shales have considerably lower D and greater CP values than other sedimentary rocks. The D of sandstone (Branlund and Hofmeister, 2008), limestone (see below), and shale (this study) at 20°C are 3.1, 1.5, and 0.8 mm2 s-1 respectively, and decrease to 1.1, 0.5, and 0.4 mm2 s-1 at 300°C. The changes in D and CP also result in a decrease in thermal conductivity (k) with increasing temperature, where k=DCPr, and r density. We are currently exploring variations in the properties of shale as a function of mineralogy and cement type (silicic, carbonate, or ferruginous).
Figure 1. Thermal diffusivity, heat capacity and thermal conductivity of selected rock types.
2. Carbonates.
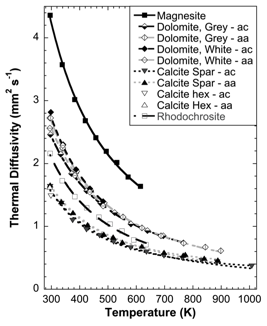
(a) Single crystals. At room temperature, D ranged from 4.36 mm2 s-1 (296 K) for magnesite, to 1.61 mm2 s-1 (300 K) for calcite. Across the range of temperatures measured for minerals, D decreased (at equivalent temperatures) from magnesite (Mg), to dolomite (CaMg), to rhodochrosite (Mn), to calcite (Ca). All minerals and rocks measured produced a marked decrease in D as temperature increased, with the strongest drop occurring in the interval ~300-500 K.
Figure 2. Thermal diffusivity of carbonate minerals as a function of temperature. For minerals measured in multiple orientations, aa ([100]) indicates heat flow parallel to the c-axis and ac heat flow perpendicular to the c-axis. Crystal orientation shows only a small influence on D at low temperature, and negligible influence at temperatures above ~500K.
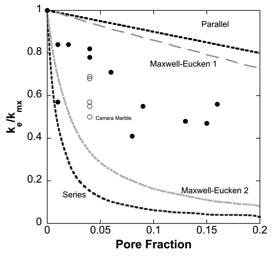
(b) Rocks. Mineralogy and porosity were also strong controls on rock D. For example, calcitic limestones showed lower D than single-crystal calcite, scaling roughly with pore fraction. Dolostone produced higher D than all cc-limestones across the interval 300-600 K. This effect was more pronounced in dolomitic marble, as D was 40-60% higher than dolostone in the same interval, and 30-50% higher than calcitic marbles in the interval 300-900 K. Additionally, for rocks, we measured heat capacity using differential scanning calorimetry, which allowed us to calculate their thermal conductivity (k = rCPD, where r is density). Our results show a stronger T-dependence for k of carbonate rocks and minerals than previous studies, with k decreasing by ~50% between ambient and ~600 K.
Figure 3. Effect of porosity on thermal conductivity of carbonate rocks. ke = effective thermal conductivity, or k including pore space. kmx = matrix thermal conductivity, or k calculated from mineral proportions without pore space. Open circles are the Carrera Marble. All samples except one fall within the boundaries of the Maxwell-Eucken model.
3. Thermal models.
For all rocks types, the decrease in D and k with increasing temperature means that the rock becomes more thermally insulating as its temperature increases.
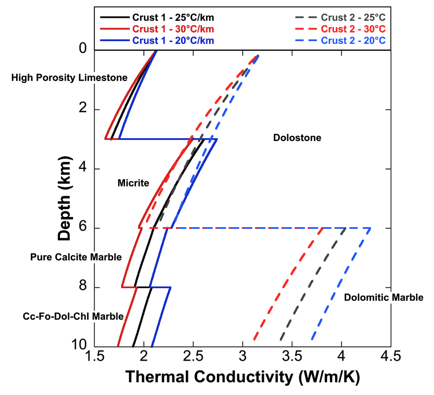
(a) The decrease in thermal conductivity with increasing temperature affects the steady-state geothermal gradient (dT/dz), because the geotherm is inversely proportional to thermal conductivity for a given heat flux. It is important to consider effects of temperature, mineralogy and texture, as well as lithology, when modeling the crust with a large proportion of carbonate rocks. Dolomitic basins will have a much higher thermal conductivity, and consequently a lower geothermal gradient, than calcite-dominated basins.
Figure 4. Thermal Conductivity profiles for 2 ad-hoc carbonate crusts calculated using linear geothermal gradients (°C/km) as indicated in the figures. Dolomitized carbonate-bearing basins (right) are more conductive than non-dolomitized basins.
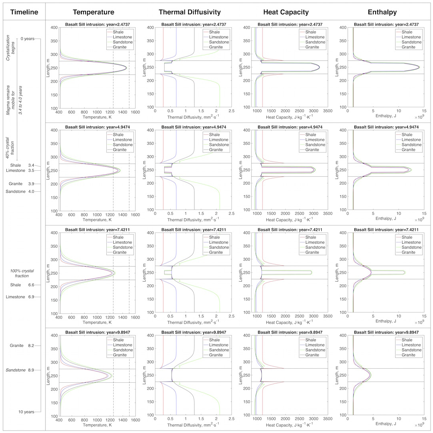
(b) The more insulating the country rock, the slower an intrusion will cool, and the longer the thermal effects of intrusions will persist. Finite difference thermal models of a basalt sill intruding granite, sandstone, limestone, and shale using the newly acquired data, suggest sills that intrude shales cool the fastest, due to the high heat capacity of shales, while the low thermal diffusivity and conductivity of shales result in a narrower thermal aureole than for other rock types.
Figure 5. Thermal models of basalt intruding different sedimentary rock types. Each row represents a different time, from ~2 to ~9 years post-intrusion. At each time, vertical profiles across the sill and its aureole are shown for (a) temperature, (b) thermal diffusivity, (c) heat capacity, and (d) enthalpy.