Reports: UNI454810-UNI4: Structure-Oxidation Potential Relationship of Antioxidants and Peroxide Formation in Gasoline
Sanela Martic, PhD, Oakland University
ACS PRF annual report S. Martic
Antioxidants are commonly used as gasoline additives in order to improve fuel efficiency and minimize the deterioration of the automotive parts. With the support from the ACS PRF, my lab proposed to 1) establish a structure-function relationship of antioxidants based on the butylated phenol core toward identifying key features of “super-antioxidants”, 2) determine antioxidant activity of butylated phenols, and 3) characterize butylated phenol additives in gasoline samples.
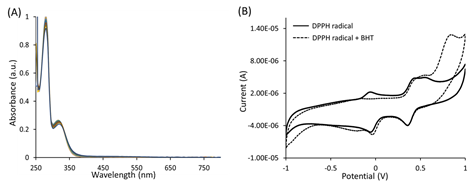
During the second year of ACS PRF support which started in Aug 2016, my group has working on objective #2 above. We started evaluating tert-butyl-phenol (2,6-di-tert-butyl-4-methylphenol (BHT)) and its analogues in terms of antioxidant activity. First, we evaluated antioxidants with superoxide anion by electrochemical methods and data indicate that all bulky phenols react with superoxide anion to similar extent. Second, all antioxidants were tested as oxidation substrate in a reaction with hydrogen peroxide and horse radish peroxidase using spectroscopy. The diaminobenzimidine (DAB) was used as an oxidation indicator which when oxidized fully exhibits absorption at ~ 465 nm. We discovered that some antioxidants, such as BHT, are better oxidation substrates than DAB (Fig. 1A). In addition, one bulky phenol reacted selectively with oxidized DAB intermediate to produce a new compound which has been identified by mass spectrometry as the major product. We are currently working on purifying the product by HPLC in order to fully characterize it by NMR. Third, we evaluated antioxidant capacity by measuring reactivity of bulky phenols with 2,2-diphenyl-1-picrylhydrazyl (DPPH•) radical. UV-vis spectroscopy was used to monitor reduction in radical level as a function of antioxidant present. We found that antioxidant capacity was highly dependent on the number and site of tert-butyl substituents. Moreover, the electrochemical methods has been used to measure oxidation/reduction of DPPH• in the absence and presence of antioxidants (Fig. 1B). The results for all three antioxidant assays are currently being included in the manuscript in preparation to be submitted to ACS J. Phys. Chem. B.
Fig. 1 (A) UV-vis spectra of BHT, DAB and HRP before and after H2O2 titration; (B) Cyclic voltammograms of DPPH• in the absence or presence of BHT.
As a part of this funding, the third project is aimed at characterizing commercial gasoline samples using the electrochemical methods. This will allow for electrochemical screening of fuel content as well as determination of electrochemical parameters, such as capacitance and resistance of the gasoline.
Four undergraduate students participated in the ACS PRF funded project (Year 2: Aug 2016-Aug 2017). Currently, one undergraduate is involved in the third project on characterization of gasoline samples by electrochemical methods.
During Year 2, the students presented one poster presentations at institutional student symposia (Oakland University). The research data acquired during the 2nd year of funding are included in the manuscript in preparation for submission to ACS J. Phys. Chem. B. PRF funding has provided undergraduates with research opportunities that are critical for their professional development and future careers. Students involved in the project have acquired research skills in analytical chemistry, electrochemistry and spectroscopy. PRF has certainly increased research productivity in my own laboratory, and allowed me to expand my research scope and interests.