Reports: DNI956822-DNI9: Polyelectrolyte-Clay Dynamics and Assembly in Taylor-Couette Flows for Applications in Improved Oil Production
Cari S. Dutcher, PhD, University of Minnesota
Annual Report Summary. We study the impact of local transient conditions on the formation, structure, and stability of polymer-clay microstructures in solution. Specifically, poly-cation flocculation of sodium-bentonite, a ubiquitous inorganic smectite clay mineral widely used in drilling fluids, is being studied in varied mixing conditions. In the work, we have performed small scale jar tests and larger scale Taylor-Couette flows to test the effects of mixing on floc growth in varied solution pH and ionic strength conditions. Additionally, a voltammetric sensor has been developed to determine the concentration of the free cationic polyacrylamide used as the flocculant, to aid in the further understanding of the kinetics and mechanisms of flocculation.
Narrative of Work. Previously, our group has used simple jar test experiments to study the effects that pH and differing ionic strengths on polymer – clay flocculation. Generally, increasing pH increases the optimal polymer dose and increases final turbidity. The ionic strength of the solution has also been varied, along with the timing of addition of salt with respect to bentonite addition. It was found that the initial bentonite aggregate size is smaller when the salt was added after the addition of the bentonite, due to sterically hindered particle growth, resulting in higher final turbidities at lower polymer doses.
While the above information provides insight into the effect of anisotropic particles in varied solution conditions, it cannot be used to directly distinguish between the mechanisms of floc growth. Additional techniques are required to understand if the observed growth rates are from floc-floc collisions or floc-bentonite collisions. In the current, ongoing work supported by the ACS PRF grant, we have used two methods to track real-time flocculation: (A) Measurements of floc size and shape during flocculation with varied hydrodynamic mixing conditions, using both jar test and Taylor-Couette flow experiments. In both flows, a laser light sheet is used to determine floc size and 2D fractal dimension over time. (B) Measurements of real-time concentration of free cationic polyacrylamide flocculant during flocculation, using voltammetric sensors.
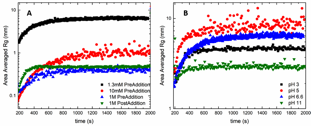
Figure 1 shows preliminary results of growth rate of the polymer – clay floc in varied ionic strength (Figure 1A) and pH (Figure 1B) conditions, using jar tests. In these experiments, polymer is added to a jar containing particle-laden water solution while mixing. Overall, increasing ionic strength decreases floc size. Also, when salt is added before bentonite (“PreAddition”), the flocs grow slower than when salt is added after bentonite (“PostAddition”) (Figure 1A).
Figure 1. Floc aggregate size as a function of time for (A) varied ionic strengths and salt addition times and (B) varied solution pH values at a total ionic strength of 1.3 mM.
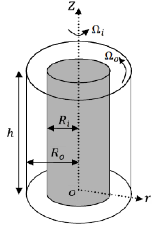
The jar tests allow for the easy adjustment of pH and ionic strength. However, the hydrodynamics in the jar is temporally and spatially inhomogeneous due to the single mixing blade that is placed in the bottom of the jar. On the other hand, Taylor-Couette (TC) flow, or flow between concentric cylinders (Figure 2), is well characterized with less spatial and temporal variation of the hydrodynamics.
Figure 2. Schematic of a Taylor Couette cell.
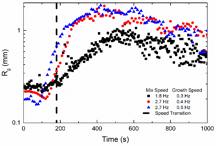
Our TC cell has injection ports built into its inner cylinder, enabling study of the entire flocculation process within the cell, which was previously impossible with traditional designs. In this study, flocculation tests were conducted within the TC cell by injecting the polymer solution into the particle-laden flows of a specific hydrodynamic flow type and turbulent intensity. After 3 min of mixing time, the speed of the inner cylinder is changed for the growth step, to mimic the procedure in the jar test. Generally, increasing the mix speed increases the growth rate of the flocs that continues into the growth step (Figure 3).
Figure 3. Floc growth rate comparison for different mixing and growth step speeds inside the TC cell.
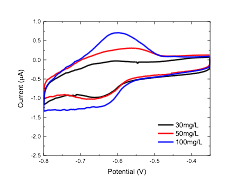
Finally, a sensor is being developed to monitor the real time concentration of free polycations in the solution, towards better understanding of the flocculation kinetics and mechanism. The sensor consists of a glassy carbon electrode base and a series of sensing membranes are applied to the bottom of the electrode surface. A potential scan will be applied to the sensor to drive the polyacrylamide into the membrane, resulting in a current peak at a characteristic potential, and the current peak height is proportional to the concentration of the polyacrylamide. The voltammetric sensor was shown to be sensitive to different concentrations of the cationic polyacrylamide by calibrating the response of the membrane to a series of polymer concentrations (Figure 4).
Figure 4. Voltammetry graph showing the different response of the voltammetric sensor when exposed to varying concentrations of the polymer of interest.
To increase the sensitivity of the voltammetric sensor at low concentrations (<30mg/L), stripping voltammetry was used to generate higher response peaks at similar concentrations. Current work includes characterization of the sensing membrane surface to improve the membrane application protocols and increase the reproducibility of the voltammetric sensor. Additionally, new sensor measurements will be taken with a gold electrode, instead of the glassy carbon electrode used previously, as they can be more clean and stable over time. Future experiments will include taking measurements of time variant polymer solutions. Ultimately, the sensor will be used to measure concentrations of the polymer flocculant in-situ during the flocculating process, providing information about the mechanisms of the initial mixing process and floc growth over time.
Impact on Career and Students. The ACS PRF grant has allowed for the funding of a first-year graduate student (Ellie Raethke, Chemical Engineering and Materials Science). While pursuing this research, the student has enriched her knowledge of fluid dynamics and electrochemistry. In addition, she has learned how to independently plan and perform scientific studies. Mechanical Engineering undergraduate researcher Ryan Bell was also supported on the ACS PRF grant, providing Ryan with his first wet lab experience. Finally, the ACS PRF grant has enhanced the PI’s career by enabling an exciting new collaboration with Chemistry Professor Phil Buhlmann, an expert in electrochemistry and ion selective electrodes, and co-advisor to Ellie.