Reports: DNI556978-DNI5: Mechanistic Studies of the Reactive Sorption of Sulfur Compounds Using Metal Oxides
Dante A. Simonetti, PhD, University of California, Los Angeles
The objectives of this project are to identify reaction models to describe reactive adsorption of sulfur compounds from gaseous streams and to interpret the physical meaning of their parameters. These objectives were advanced by studying the reaction of CuO with H2S using fixed bed experiments and in-situ X-ray Absorption Spectroscopy (XAS). The major findings during the first year of research were identification of conditions in which linear driving force models predict sorption performance and elucidation of differences in the intrinsic rate for CuO samples derived from different synthesis methods. This knowledge is important because it can be leveraged to improve the efficiency of existing sorption processes and to provide a framework for designing new materials. Furthermore, the fundamental knowledge derived from this research can be applied to a broad range of reactions and materials.
Fixed bed experiments were carried out using a commercial CuO-based sorbent. Breakthrough data (effluent H2S concentration versus time) from these studies exhibited a sickle shape, indicating that a first order rate expression (in CuO concentration) describes the sorption rate in the material balance across the bed. The resulting model consists of a single parameter (the rate constant, k), and values for k can be regressed from the breakthrough data.
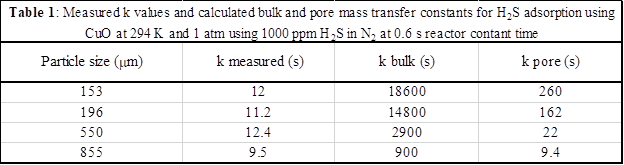
The effects of solid adsorbent pellet size on the value of k were studied to identify its physical meaning. Previous studies showed that the inverse of k represents the sum of the characteristic times for bulk diffusion, pore diffusion, and reactive sorption. For small pellets (153-196 mm), contributions from pore (162-259 s-1) and bulk diffusion (14,000-18,000 s-1) are negligible compared to the experimentally measured values of k (11.2-12.2 s-1). For larger pellets (550-855 mm) pore diffusion rates become significant (9.4-22 s-1) compared to the measured k values (9.5-12.3 s-1), and pore diffusion becomes the dominant mechanism that controls the rate of H2S removal for the largest pellet tested (measured k = 9.5 s-1; pore diffusion rate = 9.4 s-1). These data indicate that for agglomerates ≥855 mm, a linear driving force model can be used to predict sorption performance if the saturation capacity is known and the value of k is calculated using existing engineering theory. No additional information about the intrinsic reaction rate or diffusivities of species through solid product phases is required. Additionally, these experiments demonstrate that for small pellet sizes (less than ~200 mm), fixed bed experiments can be used to measure a rate parameter that only includes reaction-diffusion phenomena at the crystallite scale.
The q and k values for a CuO sample prepared in the PI’s laboratory was also measured using fixed bed experiments and compared to the values for the commercial sample. The commercial sample exhibited a higher capacity (12 wt%) compared to the lab sample (6.0 wt%), however, the lab sample exhibited a 10-fold higher k value (4.3x10-5 vs 4.0x10-6 s-1). This data indicates that differences in intrinsic surface reaction rates and/or atomic diffusivities between the two samples (that cannot be distinguished from fixed bed experiments and linear driving force rate equations) leads to slower uptake kinetics for the commercial sample which, in turn, leads to higher conversion of CuO to CuS.
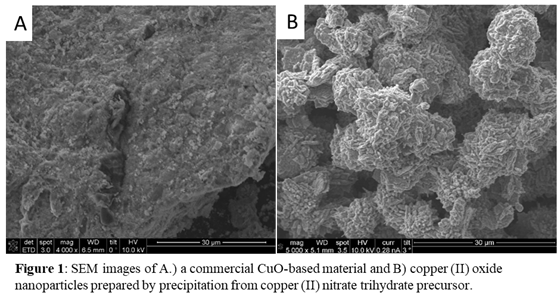
The samples were characterized using various techniques and studied using in-situ XAS to further probe the intrinsic kinetics of the reaction. Analysis of the pore structures (from N2 physisorption data) reveal similar pore volumes and surface areas for both samples. Scanning Electron Microscopy (SEM) images (Figure 1) of the lab sample revealed particles that are 1-5 mm in diameter, and these particles consist of agglomerates of <1 mm particles. The commercial sample also consists of 5-15 mm sized particles, which appear to also be agglomerates of ~1 mm particles. X-ray diffraction (XRD) patterns for both samples show characteristic peaks for CuO, and analysis of the patterns show that the CuO crystallite size of the commercial sample (3 nm) is smaller than that for the lab sample (17 nm). These characterization data indicate that the CuO samples studied are chemically and structurally similar, and the only differences are crystallite size.
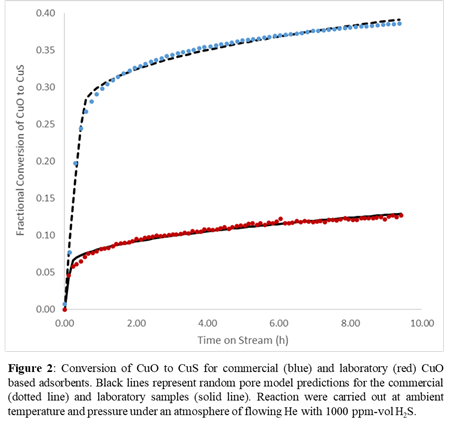
The sulfidation kinetics of the commercial and laboratory samples were measured using in-situ XAS. Linear combination fitting (LCF) was used to determine the extent of CuO conversion as a function of time from the change in the XANES spectra. The conversion-time data (Figure 2) were analyzed using the random pore model to obtain values for reaction rate constants and effective diffusivities. The rate constant for the laboratory sample (5.5x10-3 cm4 mol-1 s-1) was higher than that for the commercial sample (3.1x 10-3 cm4 mol-1 s-1). This analysis also revealed that the effective diffusivities for the two samples were similar (5.8x10-13 vs 4.2x10-13 cm2 s-1). This difference in reaction rate constant is an unexpected result given that both samples are crystalline CuO. A variety of copper sulfide products can form (CuxSy with 1≤x/y≤2) from reaction of CuO with H2S, however, the model assumes only formation of CuS. The different intrinsic reaction rates for the two samples suggests that assuming only a single product may be insufficient to full describe the fundamental mechanism of the CuO/H2S reaction and that additional techniques are necessary to identify and characterize the sulfide products so that a microkinetic model that includes all possible reactions can be constructed.
The results from this work have generated collaborations, publications, and future proposals for the PI and have also provided mentorship opportunities. The results form the basis of 4 papers that are currently in preparation and anticipated to be published during the second reporting period. These results also served as preliminary data for proposals to fund extensions of this project. This work was also granted facility time for 2016-2018 at the Stanford Synchrotron Research Laboratory to conduct XAS experiments. The collaboration with SSRL is significant because it expands the PI’s expertise and also signifies the broader value of this research. The results from this project also contributed toward the degree progress for two PhD students and provided research experiences for two undergraduate students.