Reports: ND1056149-ND10: Monolithic Composites of Metal-Organic Frameworks with Aerogels/Xerogels for Adsorbed Natural Gas Vehicles
Sridhar Komarneni, PhD, Pennsylvania State University
Metal organic frameworks (MOFs) have very high surface areas which is beneficial to vehicular natural gas storage (1-3). However, MOFs do not pack well in a fuel tank, which means interparticle pore volume will be useless for adsorption of natural gas. This interparticle pore volume could be used for methane adsorption by forming monolithic MOF composites with hydrophobic silica gels of high surface area (4). Thus, the objectives of this research are to synthesize monolithic composites of MOFs by forming hydrophobic silica aerogels in the interparticle volume of MOFs and to investigate their methane adsorption capacities on a volumetric basis.
Preparation of MOFs and aerogel solution
Two different MOFs, which are Cu3(benzene-1,3,5-tricarboxylic acid)2 (Cu-MOF) and Cr3F(H2O)2O(benzene-1,4-dicarboxylic acid)3, (Cr-MOF) were used. Cu-MOF was prepared hydrothermally at 75 oC for 24 h according to a previous method (5). Cr-MOF (MIL-101) was synthesized hydrothermally at 200 oC for 24 h based on literature (1). Precursor solution for aerogel was prepared with methyltrimethoxysilane using an acid-base catalyzed method reported previously (6).
Synthesis of monolithic MOF aerogel composites
2 ml of aerogel solution was added to1.4235 g of Cu-MOF degassed at 180 oC for 15 h or 0.5695 g of Cr-MOF degassed at 150 oC for 15h to a plastic tube (4"L×0.25" ID) and packed up to 3.25" length of the tube. After the tube was sealed by aluminum foil and parafilm, the sample was aged for 1day at room temperature and then the covered films were removed. After the composite was dried at room temperature for 1day in the tube, it was removed from the tube and then dried at room temperature for 2days followed by drying at 50 to 85 oC at 10 oC/h. During drying, 15.4% volume of Cr-MOF composite was reduced by shrinkage while no shrinkage was found in Cu-MOF composite. Like MOF samples the formed MOF monolithic composites showed original colors as seen in Fig. 1.
Fig.1
Characterization
X-ray diffraction (XRD) patterns were used to identify phases and nitrogen adsorption and desorption isotherms were measured for surface area determination.
Methane adsorption
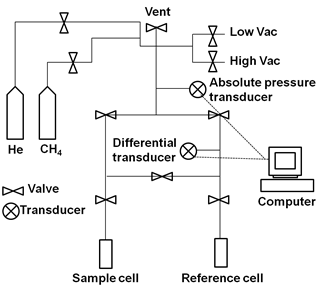
For methane adsorption to 35 bars, a custom built volumetric differential Sieverts apparatus was used to measure the methane pressure differential between adsorbing and reference (ballast) sides using an absolute and differential pressure transducer (Fig. 2) (7). A blank correction was done using He to reduce any error. Prior to analysis, Cu-MOF/aerogel composite and Cr-MOF/aerogel composite were degassed at 180 and 150 oC, respectively. For measurement of MOFs 1.4235 g of degassed Cu-MOF or 0.5695 g of Cr-MOF was packed into a sample cell to 0.544 g/ml of bulk density for Cu-MOF or 0.218 g/ml for Cr-MOF to keep the same volume as their MOF composites. A reference cell was filled with Copper turnings which do not adsorb methane and helium gas.
Fig. 2.
Results
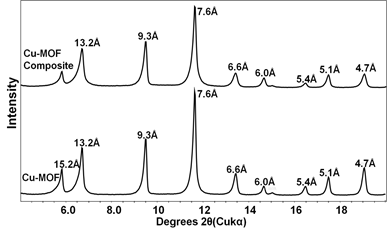
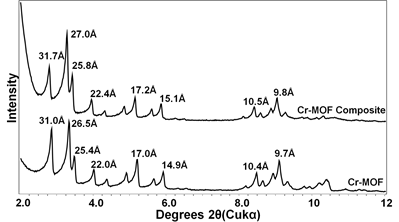
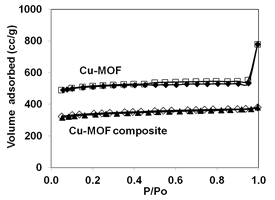
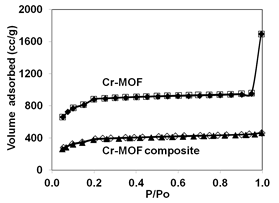
The XRD patterns of MOFs (Figs. 3 and 4) confirmed the formation of Cr- and Cu-MOFs (1, 5). Both the monolithic MOF composites showed same patterns as original MOFs suggesting they still maintained their original crystal structures. The N2 adsorption isotherms indicated all the samples are microporous showing typical Type I shape (Fig. 5). The BET surface areas of Cr-MOF and Cu-MOF were 2800 and 1552 m2/g, respectively but their composites showed lower surface areas because of aerogel (Table 1).
Fig. 3.
Fig.4.
Figure 5. Nitrogen adsorption (filled symbols)-desorption (open symbols) of MOFs and their composites.
Table 1. BET surface areas of MOFs and MOF composites.
| Cu-MOF | Cu-MOF composite | Cr-MOF | Cr-MOF composite |
BET surface area (m2/g) | 1552 | 1007 | 2800 | 1215 |
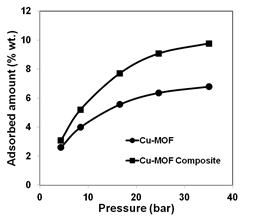
Composites of MOF and silica aerogel were prepared to increase their methane uptake capacity on a volume basis by filling in the void space between MOF particles with aerogel while keeping the volume of the MOF and composite constant. Methane adsorption isotherms were measured at room temperature up to 35 bars. As the applied pressure increased, the methane adsorption increased in all of the samples (Fig. 6). Also, both the MOF-aerogel composites showed higher capacities than MOFs only. Among the four materials, Cu-MOF aerogel composite showed the highest adsorption (9.8 wt.%) although the capacity of Cu-MOF itself is much lower (6.8 wt.%) on a constant volume basis. However, it was found that there was only a small difference in methane adsorption capacities between Cr-MOF and its composite with aerogel but further studies are in progress to determine the cause of this small difference.
Figure 6.
Our results suggest methane adsorption capacity of MOFs was improved when they were packed in the form of a monolithic aerogel MOF composite. Thus, natural gas storage could be increased in a natural gas vehicle using a MOF composites rather than MOFs. This research is very helpful to my postdoc who participated in it to pursue a career either in industry or academia. This funding helped the PI to carry out research in his field.
References
1. G. Férey, C. Mellot-Draznieks, C. Serre1, F. Millange, J. Dutour, S. Surblé, I. Margiolaki, A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area Science, 2005, 309, 2040-2042.
2. K. Koh, A.G. Wong-Foy and A. J. Matzger, A Porous Coordination Copolymer with over 5000 m2/g BET Surface Area J. Am. Chem. Soc., 2009, 131, 4184-4185.
3. M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O'Keeffe, O.M. Yaghi, Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage Science 2002, 295, 469-472.
4. V.C. Menon, S. Komarneni, Porous Adsorbents for Vehicular Natural Gas Storage: A review, 1998, 5, 43-58.
5. X. Yan, S. Komarneni, Z. Zhang, Z. Yan, Extremely enhanced CO2 uptake by HKUST-1 metal-organic framework via a simple chemical treatment, 2014, 183, 69-73.
6. B. Li, X. Gao, X. Li, Z. Liu, N. He, Monolithic organosilica aerogel consisting of hollow microspheres by a simple ambient pressure drying process, 2017, 199, 21-23.
7. S. Sircar, C.Y. Wang, A.D. Leuking, Design of high pressure differential volumetric adsorption measurements with increased accuracy, 2013, 19, 1211-1234.