Reports: UNI654592-UNI6: A Physicochemical Exploration of the Diffusion of Small Molecules in Glassy and Highly-Viscous Materials
Andrew J. Huisman, PhD, Union College
Compound |
Parameter |
Union College EDB |
PEG 3 |
p0 (Pa) |
(9 ± 1) x 10-3 |
∆Hvap (kJ / mol) |
73 ± 4 |
|
PEG 4
|
p0 (Pa) |
(1.7 ± 0.2) x 10-3 |
∆Hvap (kJ / mol) |
70 ± 2 |
Table 1: Values for PEG3 and PEG4 values from the Union College EDB, as published in Krieger et al.
Compound |
Parameter |
Union College EDB |
Krieger et al. AMTD 2017 |
PEG 5 |
p0 (Pa) |
(8.1 ± 0.2) x 10-4 |
(5.3 ± 0.8) x 10-4 |
∆Hvap (kJ / mol) |
95 ± 1 |
90.6 ± 1.1 |
|
PEG 6
|
p0 (Pa) |
(4.2 ± 0.3) x 10-5 |
(3.1 ± 0.6) x 10-5 |
∆Hvap (kJ / mol) |
100 ± 1 |
102.1 ± 1.5 |
Table 2: Comparison of PEG5 and PEG6 values from the Union College EDB to Krieger et al. benchmark values.
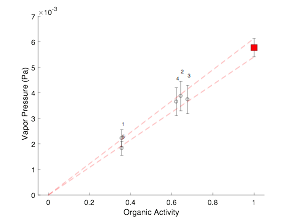
Figure 1: Measurements of vapor pressure of PEG4 as a function of organic activity, following a modified Raoult’s Law behavior. Experimental points indicated by open circles with error bounds given by error bars; the filled square point is the extrapolated value of the saturation vapor pressure of the neat liquid. |
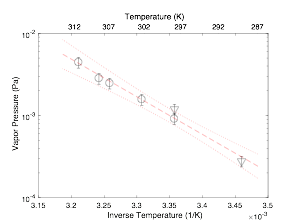
Figure 2: Measurements of the saturation vapor pressure of neat PEG5 as a function of temperature. The red dashed line is the trend line to guide the eye; dotted lines indicate the 2σ error bounds. |
References: Krieger, U. K., Siegrist, F., Marcolli, C., Emanuelsson, E. U., Gøbel, F. M., Bilde, M., Marsh, A., Reid, J. P., Huisman, A. J., Riipinen, I., Hyttinen, N., Myllys, N., Kurtén, T., Bannan, T., and Topping, D.: A reference data set for validating vapor pressure measurement techniques: Homologous series of polyethylene glycols, Atmos. Meas. Tech. Discuss., https://doi.org/10.5194/amt-2017-224, in review, 2017.