Reports: UNI656406-UNI6: Spectroscopic Investigation of Cyano Functionalized Combustion Intermediates
Paul Raston, PhD, James Madison University
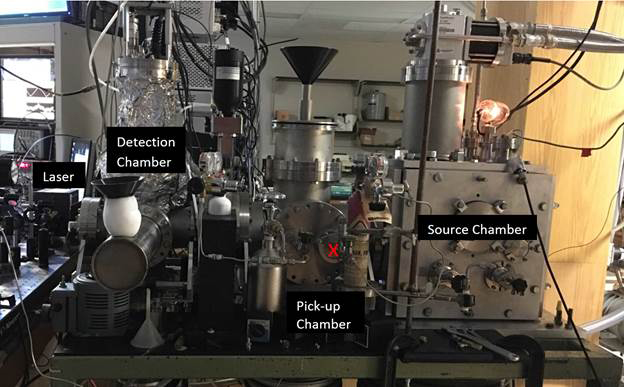
Superfluid helium nanodroplets provide a unique medium for the study of chemical dynamics at the molecular level. Their low temperature (0.4 K), enormous heat conductivity, and weakly interacting nature allows for the trapping of chemical intermediates that are typically not accessible by conventional synthetic means. The end-goal of this project is to isolate the cyano radical (CN) and to react it with stable molecules such as oxygen. The first step in this endeavor was to assemble a helium nanodroplet spectrometer, which has been completed (see figure 1). It was built by two undergraduate students over the course of the 2016-2017 academic year, and its sensitivity was optimized during the summer of 2017.
Figure 1: JMU Helium Nanodroplet Isolation Spectrometer. The x marks the intended location of the pyrolysis source (figure 3).
The top trace in figure 2 shows the spectra of OCS embedded in helium nanodroplets in the C-O stretching fundamental (n’ = 1000), which was monitoring while optimizing the sensitivity of the spectrometer. This allowed for the observation of its weak R(4) transition (which has not been observed before), as well as the first observation of the third overtone of the O-C-S bending vibration (n’ = 0400; lower trace in Figure 2). This overtone band is ~180x weaker than the fundamental, with an infrared intensity that is ~0.5x that for the fundamental of CN. The JMU helium nanodroplet isolation spectrometer is thus sensitive enough to detect CN.
Figure 2: Spectra of the C-O stretching fundamental (top) and O-C-S bending overtone (bottom) of carbonyl sulfide embedded in helium nanodroplets.
We are currently working with our machine shop to build a pyrolysis source that should be capable of temperatures up to 2000 K (see figure 3). We plan to have it completed in Fall 2017 so we can start measuring rovibrational spectra of CN in helium nanodroplets, as well as its reaction products following addition of O2 or small hydrocarbons (e.g. C2H2) to the CN doped droplets.
Figure 3: Schematic of the pyrolysis source that will be used to generate the cyano radical. It consists of an alumina tube with a resistively heated tungsten filament at the end. Collisions of BrCN with the tube walls (at 1400 K) result in dissociation to yield Br and CN.
Throughout the course of the above research, we have investigated the far-infrared spectra of vinyl-alcohol, which is (also) an important molecule in combustion. The objective is to map out the infrared bands of the higher energy anti rotamer, which we have completed in part using data that was collected at the Australian Synchrotron. We plan to complement that data with helium droplet spectra: Using the above pyrolysis source, we can increase the population of the anti rotamer, and then we can freeze it out in helium nanodroplets so that we can measure its rovibrational spectrum with good S/N. Initial results have been published in ACS Earth and Space Chemistry.
The PRF UNI grant has had a large impact on undergraduate research being performed at James Madison University. During the summer of 2017 two students were supported, and expendable supplies were purchased, which made possible the completion and optimization of the helium nanodroplet spectrometer. Students were involved in all aspects of the experimental set-up, including installing the laser system, bolting together flanges/vacuum chambers, vacuum testing, building a radiation shield (around the cold head), and implementing a sophisticated interlock network. This spectrometer is in use several times a week during semester, during which time we are routinely collecting publishable spectra. The impact this grant has already had on my career is therefore quite large.