Reports: DNI556989-DNI5: Identifying Structure-Activity Relationships for the Dehydrogenation of Alkanes on Oxides
Ioannis Bourmpakis, University of Pittsburgh
Recent work from the PI’s lab on alcohol dehydration chemistry has shown that both Lewis1-3 (LA) and Bronsted3 (BA) acids catalyze these reactions and that the acid-catalyzed dehydrogenation (DH) of alkanes exhibits similarities with elimination of beta-hydrogens (βH) by sites that are active in alcohol dehydration4. Alkane DH utilizes metal oxides as catalysts, with a concerted DH mechanism being recently proposed on acid-base site pairs on metal oxide surfaces4. Additionally, zeolites offer versatility in alkane/alkene separations, utilizing intra-pore cation exchange for selective adsorption of alkenes by specific interactions of cations and alkene pi-systems. We have highlighted the potential of cation-exchanged zeolites in hydrocarbon separations in a recent publication5.
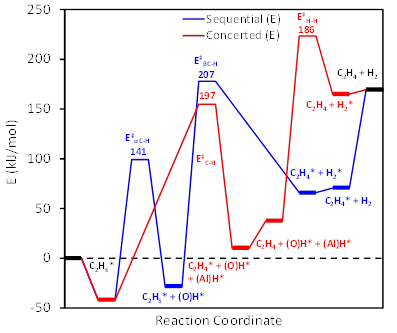
Figure 1: Energy profiles of ethane DH via the concerted (red) and sequential (blue) pathways. Adsorbed states are denoted with asterisks and transition states with double daggers.
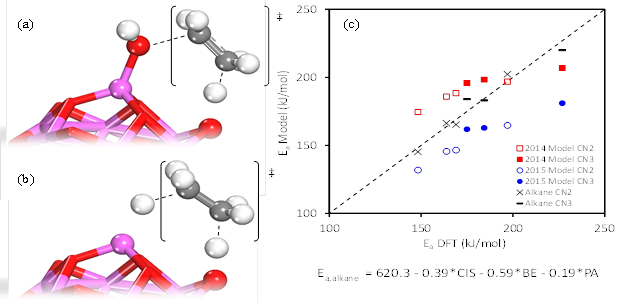
Our most recent work has focused on elucidating the surface mechanism of alkane DH pathways on aluminum oxide, using DFT calculations. As shown in Figure 1, a concerted mechanism is energetically preferred for alkene formation, with a carbenium-ion-like transition state forming on surface acid-base sites. The two alkane C-H bonds dissociate heterolytically between the surface Al – O site pairs in a single step, directly forming alkenes and surface-bound hydrogen dissociated between the two sites. A DH model was developed, quantifying acidity/basicity of the oxide surface and stability of reacting hydrocarbons as reactivity descriptors. The model was developed using methodology previously applied in structure-activity relations (SAR) for alcohol dehydration on oxides. Carbenium Ion Stability (CIS), shown to be a descriptor in alcohol dehydration, was used as a descriptor in alkane DH and was found to correlate with the calculated activation energy barriers for the hydrocarbons in question. Increased hydrocarbon substitution was found to decrease the calculated reaction barriers, based on the CIS at the transition state. Finally, SARs developed for alcohol dehydration on various metal oxides were found to accurately capture the trends in alkane DH, accounting for catalyst acid-base surface properties and CIS of intermediates at the transition state (Figure 2(c)).
Figure 2: Graphical representations of concerted elimination transition states for a) alcohol dehydration and b) alkane DH, between acid (Al) and base (O) site pairs. c) Parity plot of DFT-calculated alkane DH barriers vs. predictions of the DH model (black) and alcohol dehydration models in references [1] (2014) and [2] (2015). Different base site coordination is visualized with different markers.
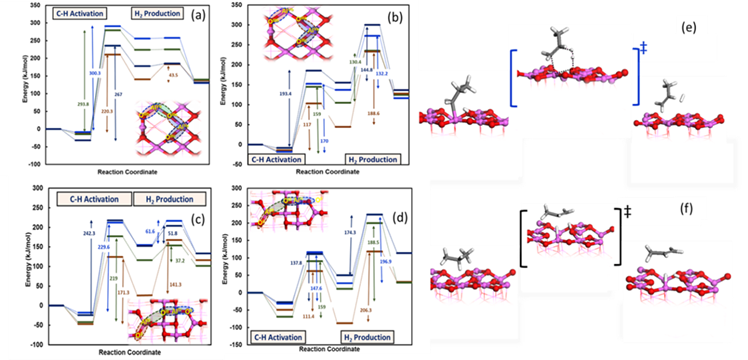
The high surface heterogeneity of γ-Al2O3, with multiple site pairs of different acid-base strength, makes it an interesting catalyst to determine the detailed mechanistic features towards developing SARs for alkane DH. We investigated the propane DH reactions on different active sites of γ-Al2O3 for two different surface facets using periodic ab-initio DFT calculations (Figure 3(a)-(d)). Two plausible mechanisms, concerted and stepwise, were evaluated for propane DH evolving over the different site pairs of γ-Al2O3.
Figure 3: Propane DH energy profile on different sites of (100) γ-Al2O3 through (a) concerted and (b) stepwise pathway and respective profiles on (110) γ-Al2O3 via (c) concerted and (d) stepwise pathway. (e) Hydrogen production mechanism through stepwise pathway and (f) C-H activation mechanism through concerted pathway.
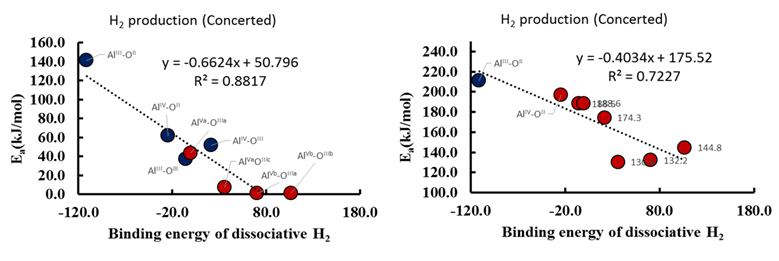
Our results demonstrated that the concerted mechanism is preferred on the low coordination number site pair (AlIII-OII) of γ-Al2O3(110), whereas the stepwise mechanism is preferred on all the other site pairs of Al2O3 on both the facets. It was noted that the rate limiting step (RLS) for the stepwise mechanism is the H2 production step (Figure 3(e)) on γ-Al2O3 (110), whereas the RLS for the concerted mechanism is the C-H activation step (Figure 3(f)) on both that facets. These findings demonstrate that the C-H activation step is not necessarily the RLS for alkane DH on metal oxides. The elucidated mechanism for the stepwise pathway suggests a direct H2 production via a six membered transition state (Figure 3(e)). The most favorable mechanism for beta-hydrogen elimination was found to be different than the commonly proposed mechanism on metal oxides (based on the CrOx catalyst). We developed propane DH SARs (Figure 4) applicable to metal oxides, and identified the dissociative H2 binding energy as a descriptor of the alkane DH activation energy. We also revealed inverse trends between the barriers of C-H activation step and H2 production step with H2 binding energies. It was revealed that DH mechanism on γ-Al2O3 is site dependent. The next step is to utilize the H2 binding energy as a descriptor to screen alkane DH on various metal oxides.
Figure 4: Propane DH barriers on γ-Al2O3 vs. dissociative H2 binding energies on corresponding acid-base sites for concerted mechanism.
The alkane DH project funded by ACS-PRF has primarily supported the graduate student, Pavlo Kostetskyy and has provided him several important insights into the current state-of-the-art understanding of complex catalytic reactions. The perspectives gained will assist him in his career development, and in part shape his upcoming career choices. Mudit Dixit (post-doc funded by the PI’s start-up), was also involved in this project and got a flourishing opportunity to explore the field of computational catalysis changing research career path (prior experience in batteries). This project has supported the undergraduate students Peter Tancini and Mitch Cholewinski (summer research for pay) and helped them realize that they greatly enjoy performing scientific research. This project has significantly aided the PI (Prof. Mpourmpakis) in i) opening a new research area in his group, that of alkane DH, ii) establishing a research group as a junior faculty and iii) starting new collaborations with experimental groups. Finally, 2 articles (ref. 3 and 5) have been published so far (both highlighted as journal covers), and two presentations have been given in scientific meetings (1 oral presentation in ACS and 1 undergraduate poster in AIChE).
References:
1. Kostestkyy P. et.al, Catal. Sci. Technol. 2014, 4, 3861
2. Kostetskyy, P. et.al, Catal. Sci. Technol. 2015, 5, 4547
3. Kostetskyy, P. et.al, Catal. Sci. Technol. 2017, 7, 2001
4. Joubert, J. et.al, Journal of Catalysis 2007, 251, 507
5. Kostetskyy, P. et.al, Ind. Eng. Chem. Res. 2017, 56, 7062