Reports: UR456516-UR4: Characterization of Ion-Radicals Pi-bonding and Electron Transfer Using Cation-Radical Salts with Weakly-Coordinating Anions
Sergiy Rosokha, PhD, Ball State University
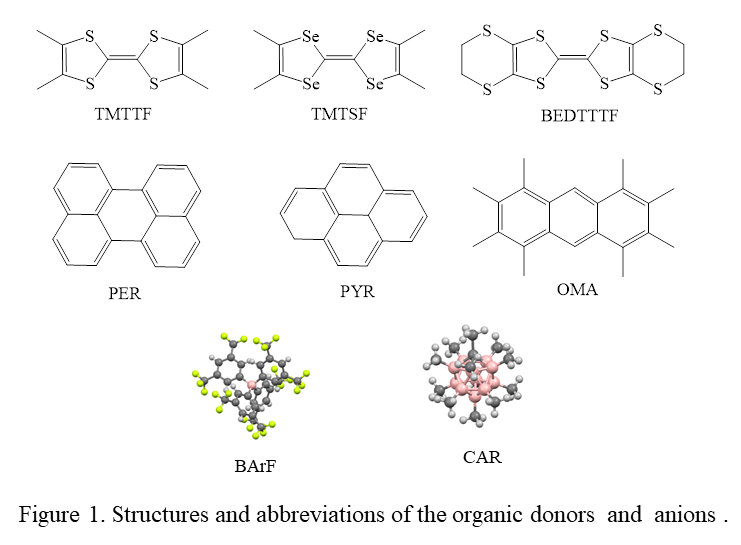
- Preparation and characterization of cation-radical salts with weakly-coordinating anions. Cation-radicals salts were prepared using the next planar organic donors (shown in Figure 1): octamethylbiphenyllene (OMB), octamethylanthracene (OMA), perylene (PER), pyrene (PYR), tetrathiafulvalene (TTF), tetramethyltetrathiafulvalene (TMTTF), tetramethyltetraselenafulvalene (TMTSF), bis(ethylenedithio)tetrathiafulvalene (BEDTTTF). Most of these donors (i.e. PER, PYR, TTF, BEDTTTF, TMTSF) are commercially available. OMA and TMTTF were prepared during preliminary studies. OMB was synthesized from 1,2-dibromotetramethylbenzene (eq 1) according to the literature procedure:
2C10H12Br2 + n-BuLi → OMB
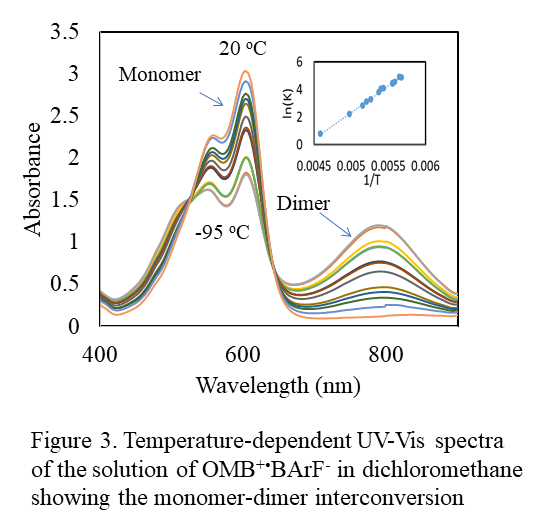
- UV-Vis spectral and X-ray crystallographic studies of formation of the p-bonded complexes. The dimerization of the cation-radicals in the presence of the weakly-coordinating anions was tested via the variable-temperature (from +20 oC to -96 oC) UV-Vis measurements of the solution of OMB+·BArF- salt in dichloromethane (Figure 3A). At room temperature, the UV-Vis spectrum of a 4 mM solution of this salt show intense absorption band of OMB+· with maximum at about 600 nm and a very weak and broad low-energy absorption. When the temperature of the solution was progressively lowered to -90 oC, the intensity of the low-energy absorption increased and the intensity of the band at 600 nm decreased. Earlier studies (with the SbCl6- anion) showed that the band with λmax = 602 nm is related to the absorption of the OMB+· monomer and the low-energy band at λmax = 792 nm is related to the (OMB)22+ dimer. The clear existence of a pair of isosbestic points established the quantitative interchange between these two species in our experiment (Figure 3).
OMB+· + OMB+· ↔ (OMB)22+
The quantitative evaluation of the concentrations of both monomeric and dimeric forms yielded the equilibrium constants KD at each temperature. The enthalpy and entropy for dimer formation, DH = -31.8 kJ/mol and DS = -140 kJ/mol were obtained from the linear temperature dependence shown in the insert in Figure 3.
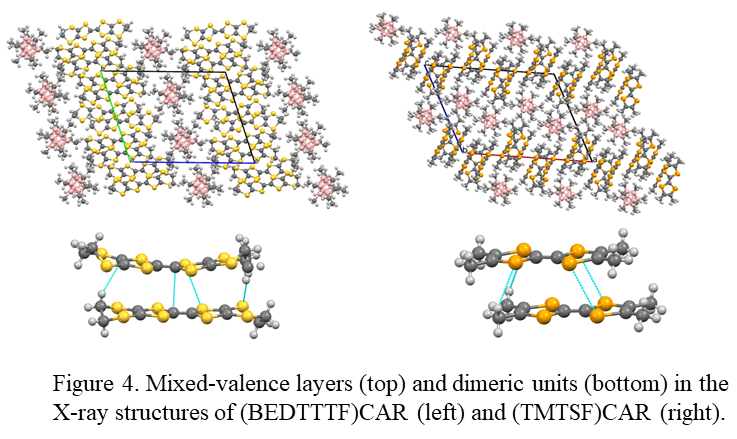
Valuable information about the cation-radicals and their π-bonded complexes could be obtained using X-ray crystallography. Earlier, we established X-ray structures of the 1:1 D+· BArF- or (Dn)+· BArF- salts with some of the donors considered in the current projects. Most of them showed 1D molecular nano-wires of mixed-valence π-bonded dimeric associates separate by the counter-ions. In the current work, we started preparations of the single crystals of D+· cation-radicals with CAR- anions. In particular, slow evaporation of the solutions of BEDTTTF+·CAR- and TNTSF+·CAR- in 10:1 dichloromethane/hexane in the presence of a large excess of neutral molecules resulted in precipitation of the single crystals suitable for the X-ray measurements. The X-ray measurements and structure determination of these crystals were carried out at Purdue University by Dr. Mathias Zeller. These measurements established that these crystals comprise mixed-valence salt (Figure 4) in which quazi-1D layers of organic cation-radicals are separated by the inert anions. Similar mixed-valence BEDTTTF and TMTSF cation-radical salts (with the other anions) showed earlier very interesting conducting (and even super-conducting) properties. Thus, we intend to prepare larger amounts of these crystals for the study of their conducting and magnetic properties. Also, these crystals comprise distinct dimeric units important for the evaluation of π-bonding.
- Student training. During 1st year, three undergraduate and two graduate students participated in the project. They were trained by the PI and subsequently carried out syntheses of organic donors, cation-radical salts and/or UV-Vis measurements. As part of their research, students were involved in the literature search and analysis of literature data and their own results. This improved their critical thinking.
In summary, during 1st year of the project: a) syntheses of the suggested cation-radicals salts with the weakly-coordinating compounds were completed in amounts sufficient for the measurements of the π-bonding (repeated syntheses to make more compounds will be carried out, as needed); b) UV-Vis spectral measurements (including low-temperature measurements of the dimer formation) and EPR measurements of cation-radicals tested using newly-purchased UV-VIS and EPR spectrophotometers; c) students were trained in synthesis and UV-Vis spectral measurements. The accomplishment of the 1st-year objectives created solid basis for the further development and successful fulfillment of the project.