Reports: DNI354907-DNI3: Synthetic Strategies to Optimize Photophysical and Photoredox Properties of Organometallic Complexes
Thomas S. Teets, University of Houston
The PI’s research group specializes in synthetic and physical inorganic chemistry, using complementary synthetic strategies to control the photophysical and photochemical properties of organometallic compounds. Much of the research involves bis-cyclometalated iridium compounds, a well-known class of complexes which exhibit efficient, color-tunable phosphorescence and in many cases and addresses three key outstanding challenges associated with these compounds and their potential applications: 1) the design of complexes with efficient emission in the extremes of the visible spectrum (deep blue and deep red), 2) the development of efficient near-infrared phosphors, and 3) the discovery of more potent photoreductants which can react with a wider range of substrates in photoredox catalysis. Support from the ACS PRF has allowed the PI and his group to establish some promising new directions and make some important initial discoveries in all three of these areas.
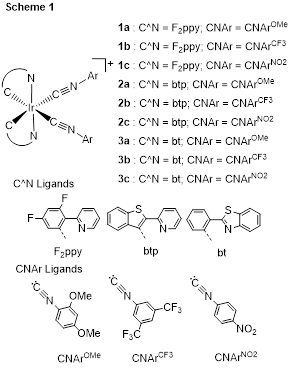
A large part of the research program seeks to identify new synthetic methods for the preparation of bis-cyclometalated iridium emitters, utilizing “on-complex” postsynthetic modification to access new structure types. The PI’s group has recently disclosed a class of bis-cyclometalated iridium complexes with ancillary aryl isocyanide ligands, which in their own right are efficient color-tunable phosphors with excellent chemical stability (see Scheme 1).
The emission spectra of isocyanide complexes 1a–3c were studied, and they were found to depend minimally on the isocyanide ligand. Blue-emitting complexes 1a and 1b and green-emitting complexes 3a–3b show efficient luminescence (Φ > 0.3), and in the case of complex 1a the electron-donating methoxy group results in a modest but significant enhancement of the quantum yield, resulting in a value of 0.50.
In more recent work these complexes have been used as reactive synthons to allow installation of unique ancillary ligand structures not obtainable by traditional routes. Bis-isocyanide complexes with electron-withdrawing groups (1b, 1c, and 1d (Ar = 4-CF3C6H4) react with hydrazine via chelative insertion, forming complexes with chelating, bis(acyclic diaminocarbene) ancillary ligands, a structure not obtainable via traditional ligand-substitution routes (Scheme 2).
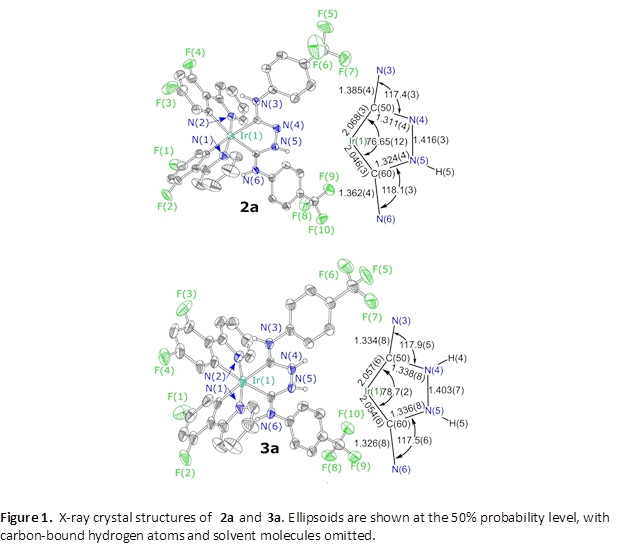
The complexes can be protonated at the nitrogen backbone to form 3a–3c, allowing access to structures in two different protonation states. Crystal structures of some of the dicarbene complexes have been determined, and as a representative example the structures of 2a and 3a are shown in Figure 1. Subtle structural changes accompany protonation of the ligand backbone, but the structure of 3a is more symmetric and includes more π delocalization than that of 2a.
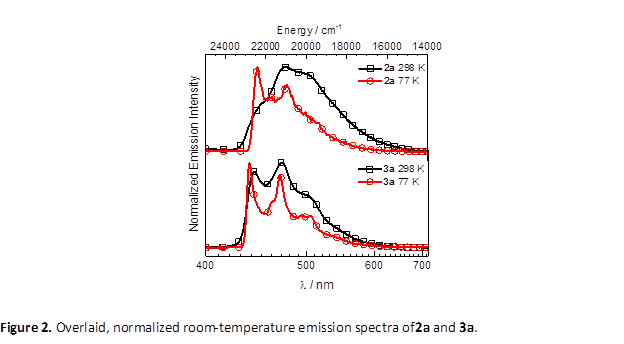
Complexes 2a/3a and 2b/3b are luminescent, and as a representative examples the emission spectra of 2a and 3a are collected in Figure 2. In complex 2a the large hypsochromic shift that accompanies cooling the sample to 77 K is indicative of significant charge transfer character in the excited state. Quantum yields for these complexes are quite low (0.044 for 2a and 0.078 for 3a), but the emission is responsive to protonation of the backbone. Protonation of the dicarbene results in a significant blue-shift in the emission, and almost twofold increase in the quantum yield, and significantly less charge-transfer character in the excited state. All of these indicate that the backbone protonation provides an additional layer of control over the luminescence and motivates pursuit of sensing platforms based on these diaminocarbene architectures.
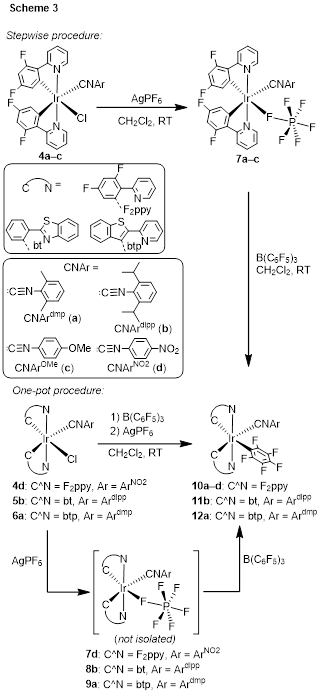
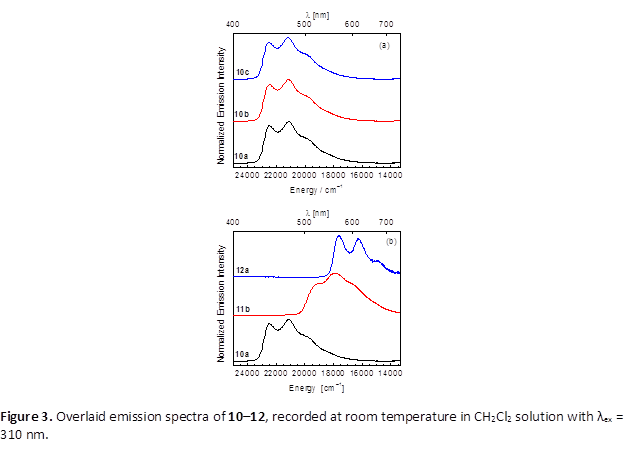
Using complexes with only one aryl isocyanide ligand and one chloride (4–6), an unusual, directing-group-free transmetalation reaction was discovered, which gives access to the first examples of bis-cyclometalated iridium complexes with unchelated aryl ligands. The reactions proceed through intermediates 7–9b, with weakly coordinated PF6– ligands, which react with B(C6F5)3 to furnish the perfluorophenylated products 10–12. The reactions tolerate a range of cyclometalating (C^N) ligands and isocyanide ligands on iridium, including sterically crowded 2,6-diisopropylphenyl isocyanide as well as electron-rich 4-methoxyphenyl isocyanide and electron-poor 4-nitrophenyl isocyanide. The perfluorophenyl complexes without nitro substituents are luminescent, with the emission color and quantum yield dependent on the cyclometalating ligand (Figure 3). Blue-emitting compounds 10a–d exhibit moderate quantum yields (0.081–0.14), green-emitting complex 11b has a high quantum yield of 0.38, whereas orange-emitting complex 12a has a very low quantum yield 0.0050. Although the photophysical properties of these compounds are not particularly impressive, the synthetic advances in this work show that transmetalation is a viable strategy for preparing novel bis-cyclometalated iridium structures, and related efforts will continue to target compounds with enhanced or unique photophysical properties.
In other unpublished work supported by PRF, bis-cyclometalated iridium complexes with nitrogen-containing β-ketoiminate ancillary ligands have been shown to be efficient red phosphors and are being incorporated into organic light-emitting diodes (OLEDs). Related complexes with electron-rich β-diketiminate ancillary ligands are potent photoreductants with thermodynamics and kinetics for photoinduced electron transfer that exceed those of state-of-the-art photosensitizers. These complementary efforts supported in part by ACS PRF have allowed some of the longest-standing challenges in the synthetic and physical chemistry of bis-cyclometalated iridium complexes to be addressed.