Reports: DNI456225-DNI4: Applications of N-Centered Radicals Generated from Electrochemical Initiation of Stable Organic Precursors
Ryan Baxter, PhD, University of California, Merced
The conversion of hydrocarbons into precious commodity chemicals remains an important and significant focus of petroleum research. In particular, radical C-H amination reactions offer the potential for refinement of complex hydrocarbon mixtures and the synthesis of value-added nitrogen-containing chemicals from feedstock materials. However, uncontrolled radical processes often limit the utility of radical reactions, and controlling the concentration of reactive species during a reaction is a challenge. In addition, many radical precursors are thermal or photosensitive, requiring special handling or storage. To address these issues, our proposed research aimed to achieve two objectives: 1) Utilize electrochemistry to control the rate of radical initiation for the generation of N-centered radicals, and 2) Develop general methods for radical amination from easily made, bench-stable radical precursors.
During the first year of funding, our research group (a) synthesized a series of nitrogen radical precursors containing activated nitrogen-oxygen bonds, (b) explored the electrochemical properties of these reagents, and (c) developed a general method for iron-catalyzed radical aryl amination from stable organic precursors.
Objective 1: Controlled Electrochemical Initiation of N-Centered Radicals
General Methods: To effectively initiate radical aminations via bulk electrolysis, we used analytical electrochemistry to identify optimum parameters for reductive cleavage of N-O bonds in various radical precursors. During bulk electrolysis, we monitored the rate of radical initiation using in situ ReactIR coupled to the electrochemical cell via a continuous flow apparatus.
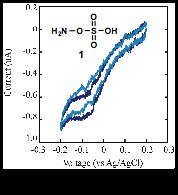
We initially began studying hydroxylamine-O-sulfonic acid (HOSA, 1) as a nitrogen-radical precursor for arene amination (Figure 1). Under aqueous conditions, HOSA displayed irreversible reduction near -0.10 V (vs. Ag/AgCl), although additional electrochemical analysis was not reproducible, owing to the poor stability of HOSA even under ambient aqueous conditions. Subsequent research focused on analyzing the electrochemical properties of more 'bench-stable' radical precursors. As shown in Figure 2, several "N-O" radical precursors were synthesized and their electrochemical properties were explored.
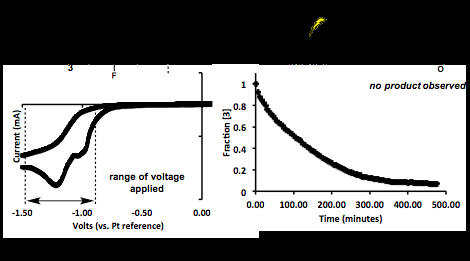
Compound 3 displays an irreversible reduction waveform near -1.06 V (vs. Pt pseudo electrode), and was chosen as a model system for bulk electrolysis based on literature precedence for succinimide radicals adding to electron-rich arenes. Based on cyclic voltammetry, we monitored the electrochemical reduction of 3 across a range of voltages. We were pleased find that we could quantify the rate of electrochemical reduction by monitoring the cleavage of the N-O bond under an applied voltage. Unfortunately, although we could reliably reduce 3 via bulk electrolysis, radical amidation of anisole was not successful under any of the conditions studied. Current efforts involve continued optimization of experimental conditions to promote radical amidation via bulk electrolysis.
Objective 2: Methods for Radical Amination from Bench-Stable Reagents
General Methods: Cyclic voltammetry was used to compare the reduction potentials of several N-centered radical precursors. Based on these results, certain reagents were targeted for reduction by simple Fe(II) catalysts and used for radical amination of electron-rich arenes.
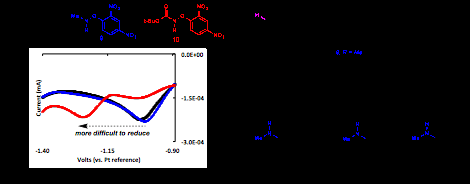
Although electrochemical initiation of radical aminations/amidations was unsuccessful, important trends in reduction potential were identified for a series of radical precursors. As shown in Figure 4, precursors containing di-nitrophenyl activating groups all showed strong irreversible reductions when studied by cyclic voltammetry. Interestingly, a significant shift in reduction potential was observed as the substitution on nitrogen was varied. Amino- (8) and methyl-amino (9) precursors showed similar values for reduction, but a tert-butyl carbamate (Boc) protecting group dramatically shifted the reduction potential to higher voltage. This change in electrochemical behavior was shown to mirror trends in reactivity for Fe(II)-catalyzed radical aminations. Radical arylation of various arenes was successful using both 8 and 9 as radical precursors. However, no product of any sort was observed from the N-Boc radical precursor. These important results suggest that reduction potentials for nitrogen radical precursors may be used as a predictive tool in the context of iron-catalyzed radical aminations.
In addition to the work described above, our electrochemical studies led us to discover unexpected reactivity in the context of silver-catalyzed radical fluorination. We found that certain nitrogen-containing additives changed the oxidation potential of simple Ag(I) salts, allowing for the transition of Ag(I) to Ag(II) using mild oxidants. We utilized this knowledge to develop a simple radical fluorination reaction that was effective in the context of simple benzylic molecules, a class of substrates important to the petroleum industry.
Conclusion
Through funding provided by the petroleum research foundation, our lab was able to establish fundamental reactivity patterns for nitrogen radical precursors. We used this knowledge to develop a simple chemical protocol for radical amination using inexpensive Fe(II) salts. In addition, through our exploratory work, we identified new modes of electrochemical and radical reactivity that has served as the foundation for new science being developed in our lab. The students supported on these projects have been afforded a unique opportunity to develop new chemistry based on the study of fundamental electrochemical and radical reactivity.