Reports: DNI656529-DNI6: Structure, Dynamic, and Solvation of a New Class of Designer Solvents by Ultrafast Nonlinear Infrared Spectroscopy
Daniel G. Kuroda, PhD, Louisiana State University
Summary
Deep eutectic solvents (DES) are a new type of designer solvent formed by a binary mixture of an ionic compound with a hydrogen-bond donor, both with melting temperatures above room temperature.[1] Due to their simple preparation and tunable properties with concentration, DES have been proposed as an inexpensive and green alternative to conventional solvents. Interestingly, simple chemical modifications to the DES components have a profound impact on properties such as fusion point, density, viscosity, etc.[1] However, the molecular arrangement and interactions among DES components giving rise to their properties is still lacking. Recently, experiments have started to show that the DES structure appears to have a high degree of heterogeneity in the nanoscopic domain.[2] Thus, a thorough characterization of the DES molecular structure and interactions will help us not only to gain a deeper understanding of the complex solvent, but also to rationalize the observed DES physico-chemical properties in terms of its microscopic structure. The overall goal of this research project is to characterize the time-scale and mechanism of the structural changes occurring in DES as a function of their structure, as well as the resulting DES solvation properties.
Objectives
Aim 1: gain insights of the structure and dynamics of DES.
Aim 2: determine the effect of choline moieties on the structure and dynamics of DES
Aim 3: determine the solvent-solute interactions and dynamics of ionic and non-ionic solutes.
Accomplished Objectives
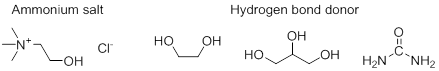
The PI and his research group has dedicated part of the project first year to studying the solvation dynamics (aim 3) of two ionic compounds (azide and thiocyanate ions) in three distinct DES using an array of experimental (IR methodologies) and computational methods.[3] The selected DES are composed of choline chloride as the quaternary ammonium salt and urea, glycerol, or ethylene glycol as the hydrogen-bond donor (Figure 1).
Figure 1. Structure of DES components. From left to right: choline chloride, ethylene glycol (EG), glycerol (G), and urea (U).
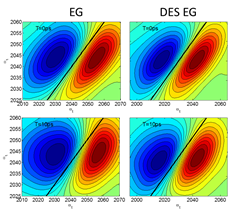
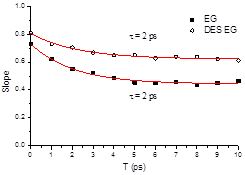
The experimental results show that the ionic solutes occupy the same places as the chloride ions in the pseudo ionic lattice of the DES since it observes the same type of interactions; i.e., hydrogen bonds with the hydrogen-bond donor. However, the solvation dynamics derived from Two Dimensional IR (2DIR) experiments show a contrasting difference between azide and thiocyanate ions. In the case of the azide ion, the solvation dynamics (Figure 2) show almost no difference between the ion in the DES EG and in its parental solvent (EG alone), indicating the azide ion is observing a similar environment in both solvents; i.e., hydrogen bonds with the hydrogen-bond donor.
Figure 2. 2DIR spectra of the azide ion in the DES EG and EG alone and the solvation dynamics derived from it (right panel).
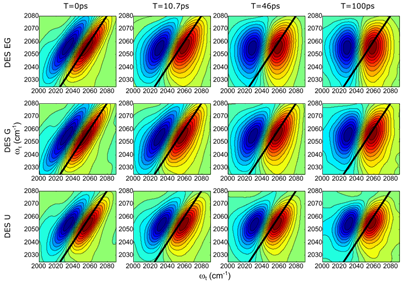
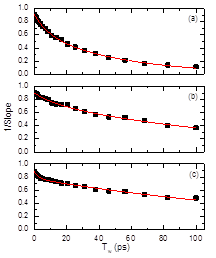
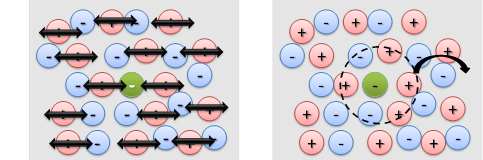
The thiocyanate ion shows much different solvation dynamics in the different DES (Figure 3). In this case, the motions of the solvent present two different time scales: a short (t < 20 ps) and long characteristic times (t > 50 ps). The difference in solvation dynamics between azide and thiocyanate ions is due to the formation of the hydrogen bonds. The thiocyanate ion hydrogen bonds through its sulphur atom, which leaves the nitrile group to sense the environment without being dominated by this interaction; while the azide ion can only do it through one or both of its nitrogen atoms. The characteristic times observed for the thiocyante ion are assigned to in-place and diffusion motions of the DES component (Figure 4). The proposed molecular mechanism for the solvation dynamics was confirmed via NMR experiments and computational studies (molecular dynamics simulations and ab initio computations).
Figure 3. 2DIR spectra of thiocyanate ion in DES EG, DES G, and DES U and the derived FFCF (right panels).
Figure 4. Cartoon representation of the molecular fast (left) and slow (right) solvent motions.
The experimental data also reveal a correlation between the slow solvation dynamics and the viscosity which is similar to what has been previously observed in ionic liquids. The results indicate that the diffusion of the components can be tailored by modifying the overall viscosity of the DES. Finally, analysis of disorder of the molecular environment of the DES through the IR lineshape reveals that alcohol based DES have a more disorganized ionic pseudo lattices than their amide analogues and that DES EG has the highest level of disorganization. While a correlation between the molecular disorganization of the solvent and the inverse molar volume is observed, a more thorough experiment with a larger sample of DES is needed to evaluate this correlation.
Ongoing Objectives
Currently and during part of year one, we have started to characterize the molecular structure of a pure DES as a function of the quaternary ammonium salt (aim I and II) for a set of amide based DES using the same experimental and theoretical methodologies. In addition, we have developed a new amide based non-ionic DES in which we will study the solvation dynamics of ionic and molecular solutes (aim III).
Conclusions
We have successfully characterized the solvation structure and dynamics of two ionic solutes in different DES using an array of IR spectroscopies and computational methods. Our results suggest that ionic solutes insert within the ionic pseudo lattice of the DES. In addition, ionic compounds observe primarily the motion of other ionic components; i.e., the quaternary ammonium cation. A molecular mechanism was proposed for explaining the observed dynamics. Finally, our studies revealed that the level of organization of the solvents is related to the hydrogen-bond donor structure. While the level of molecular organization of the DES seems to have a correlation with the molar density of the DES, more studies are needed to assess the validity of this correlation.
References
[1] Smith, Emma L., Andrew P. Abbott, and Karl S. Ryder. Chemical reviews 114, no. 21 (2014): 11060-11082.
[2] Kaur, Supreet, Aditya Gupta, and Hemant K. Kashyap. The Journal of Physical Chemistry B 120, no. 27 (2016): 6712-6720.
[3] Cui, Y., K. D. Fulfer, J. Ma, T. K. Weldeghiorghis, and D. G. Kuroda. Physical Chemistry Chemical Physics 18, no. 46 (2016): 31471-31479.