Reports: UR656597-UR6: Spectroscopic and Theoretical Characterization of Radical Lone Pair-Pi Complexes
Jay C. Amicangelo, Penn State Erie, The Behrend College
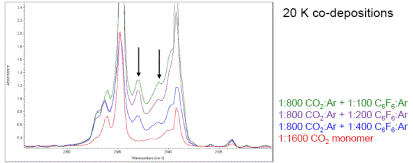
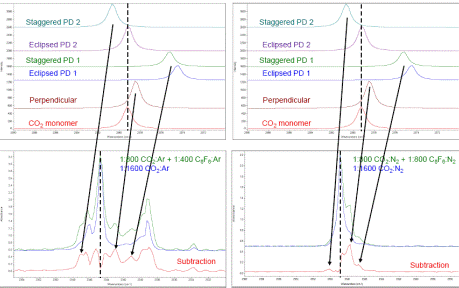
Progress towards the goal of characterizing radical lone pair-pi complexes during the first year grant period of July 1, 2016 to August 31, 2017 was a bit hampered due to two factors: the first that I was not able to recruit any undergraduate students to work in my lab during the fall 2016 semester and an equipment failure that occurred during the 2017 summer. During the spring 2016 semester, I had one senior undergraduate chemistry major and two junior undergraduate chemistry majors working in my research group. That semester we were working on characterizing the infrared spectrum of a lone pair-pi complex between carbon dioxide (CO2) and hexafluorobenzene (C6F6) in low temperature argon matrices (CO2-C6F6). We performed a series of experiments in which we deposited mixtures of CO2/Ar and C6F6/Ar at 20 K over a range of sample:matrix (S:M) ratios from 1:100 to 1:800. By comparing the co-deposition spectra with the spectra for each individual monomer, a set of new infrared peaks that I attributed to the CO2-C6F6 complex were observed, particularly in the O-C-O antisymmetric stretching region. Example spectra with the peaks attributed to the CO2-C6F6 complex indicated by arrows are shown below.
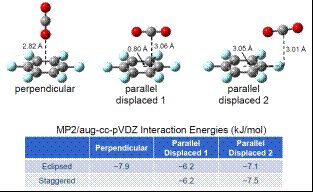
My senior student graduated and the two junior students (a non-traditional married couple) had other employment for the summer and did not work in my lab over the summer of 2016. When the fall 2016 semester began, the two junior students unfortunately decided not to return to Penn State Erie and I was not able to recruit any other undergraduate students to work in my lab during the fall 2016 semester. Therefore, I was unable to perform matrix isolation experiments during the fall semester. What I did work on during the fall semester was an extensive series of potential energy surface calculations at the MP2/aug-cc-pVDZ level of theory for the CO2-C6F6 complex system, in which I performed rigid scans along the intermolecular distance and various angle coordinates for several general orientations of the two monomers. Based on these calculations, I was able to perform full geometry optimizations and find three minimum energy configurations for the CO2-C6F6 system, in which the CO2 is either in a perpendicular or parallel orientation with respect to the C6F6. Examples for the eclipsed orientations are shown below along with the predicted interaction energies.
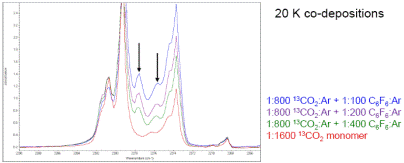
During the spring 2017 semester, I was able to recruit an undergraduate biology major to work in my lab and we performed additional matrix isolation infrared spectroscopy experiments on the CO2-C6F6 system in argon matrices but using carbon-13 isotopic carbon dioxide (13CO2). We performed a series of co-deposition experiments with 13CO2/Ar and C6F6/Ar mixtures at 20 K with S:M ratios ranging from 1:100 to 1:800 and we were able to observe peaks that could be attributed to the isotopic 13CO2-C6F6 complex. Example spectra are shown below.
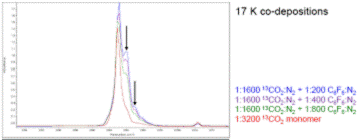
During the summer of 2017, I was able to recruit a junior undergraduate chemistry major to work in my lab and for the first part of the summer we continued the experiments for the CO2-C6F6 complex using CO2 and 13CO2 but in low temperature nitrogen matrices at 17 K. Similar to the argon matrix experiments, we performed the nitrogen experiments with a range of S:M ratios (1:200 – 1600) and we observed peaks attributable to the 13CO2-C6F6 and CO2-C6F6 complexes. Example spectra in nitrogen matrices are shown below.
Upon comparing the relative peak positions for the experimental and theoretical infrared spectra (shown below), we were able to assign the observed complex peaks to specific predicted minimum structures of the CO2-C6F6 complex. In addition to the two CO2-C6F6 complex peaks marked with arrows in the spectra presented above, which we assigned to the perpendicular and parallel displaced 1 structures, we were also able to identify another weak intensity complex peak that we have assigned to the parallel displaced 2 structure. I presented this work at the 2017 International Symposium on Molecular Spectroscopy conference in June 2017.
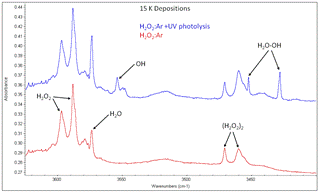
When I returned from the conference, my plan was that we would focus for the remaining months of the summer on trying to produce and characterize the complex between the hydroxyl radical (OH) and C6F6, which was one of the radical lone pair-pi complexes I had proposed studying for the grant project. To produce the OH radical, I was planning to try a few different methods, all of which require the use of our 2450 MHz microwave power supply, such as vacuum-ultraviolet photolysis of H2O, microwave plasma discharge of H2O, and microwave plasma discharge of H2 and O2 mixtures. However, when we tried to perform the first experiment utilizing the microwave power supply, we discovered that the power supply was no longer working properly and was not producing microwave radiation (it was last used in 2013). While I was trying to diagnose the problem of my instrument technician, to keep moving forward with some kind of matrix isolation experiments, we decided to perform some co-deposition experiments with ammonia (NH3) and C6F6, to attempt to characterize the infrared spectra of the lone pair-pi NH3-C6F6 complex and we were able to observe some peaks that I have tentatively assigned to this complex. After we completed these experiments, I found a literature article that described the production of the OH radical from argon matrix deposited hydrogen peroxide (H2O2) using ultraviolet radiation (200 – 300 nm) and we then began working on trying to produce the OH radical this way. Just obtaining good quality argon matrices with H2O2 proved to be challenging, however, we have been able to overcome the experimental challenges and have been able to produce good quality argon matrices with H2O2 and have also been able to observe peaks due to the hydroxyl radical and the hydroxyl complex with water (H2O-OH). An example of the spectra obtained are shown below. I am still working on repairing the microwave power supply and I am confident that I will be able to produce and observe the OH-C6F6 complex during the next grant year period.