Reports: ND156849-ND1: Organotrifluoroborates as Fluoride Ion Sources in Cationic Fluorination for the Preparation of Fluorinated Organics
David M. Perrin, Ph.D., University of British Columbia
Fluorine is unknown in feedstocks commonly produced by the petrochemical industry yet features prominently in molecules of high added value for agrochemicals and pharmaceuticals. There is therefore an urgent need to develop environmentally friendly fluorination processes, finding fruitful extension in flow chemistry. While there is surging interest in new methods including elegant metal-catalyzed reactions new applications of electrophilic fluorinating reagents, most industrial processes still rely on either halogen exchange (halex) processes, use of nucleophilic fluoride or HF in addition reactions, or the Balz-Schiemann reaction. The latter is of interest for both fine-chemical and process applications. Nevertheless, generally harsh conditions and high temperatures are required to react a diazonium-tetrafluoroborate salt (ArN2+BF4-).
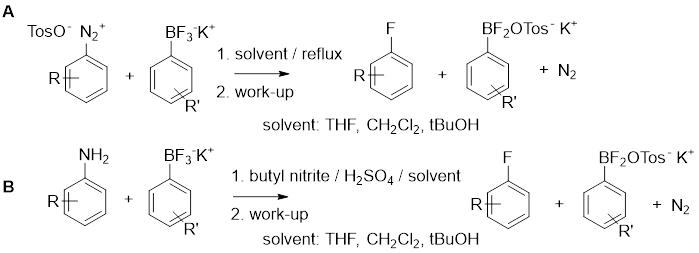
Our working hypothesis, that we formulated based on promising preliminary data, is that organotrifluoroborates can serve as sources of fluoride ion for nucleophilic addition onto an electron deficient carbon centre that is created via a dediazoniation of a diazonium ion (Figure 1).
Figure 1: A) modified Balz-Schiemann reaction starting with a diazonium salt; B) modified Balz-Schiemann reaction where the diazonium salt is prepared in situ.
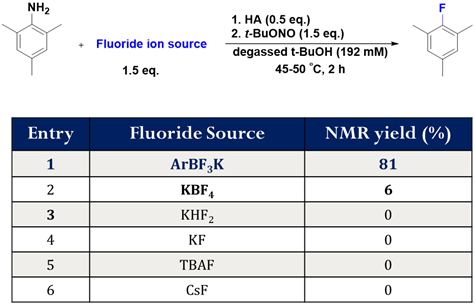
In the first fiscal year of this PRF New Directions Grant, funds supported the salaries of one postdoctoral fellow, one graduate student and one summer undergraduate student. Together these trainees clearly confirmed this hypothesis starting with trimethylaniline; in this preliminary finding we showed that with certain conditions, only the ArBF3 can support dediazoniative fluorination following in-situ diazonium ion creation.
Figure 2: Demonstration of high yielding fluorination in the presence of the ArBF3 following in situ diazotization; under the same conditions no other fluoride source supports fluoro-dediazoniation.
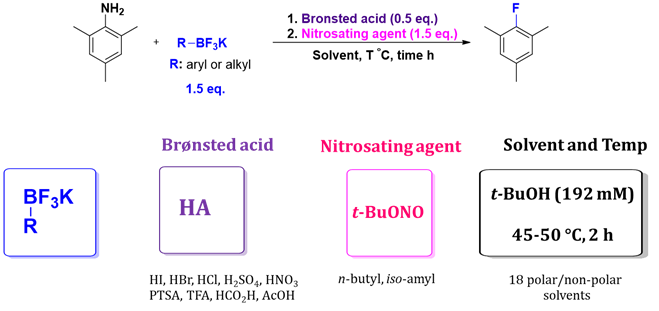
To continue this line of investigation, we sought to test numerous conditions, organotrifluoroborates, and diazonium salts, as shown schematically in Figure 3.
Figure 3: Schematic overview of optimization effort; testing various organotrifluoroborates, Bronsted acids, solvents, temperatures, reaction times in the presence of t-BuONO as the preferred nitrosating agent.
In a preliminary optimization effort, we tested various organotrifluoroborates (aryl and alkyl) for their ability to serve as fluoride anion donors under conditions of reaction shown in Figure 2. Furthermore, we procured a significant number of anilines either from commercial sources or via nitration and subsequent reduction in the presence of either H2 on Pd/C or by treatment with Ra-Ni. Once in hand, these were diazotized in-situ by treatment with t-BuONO in the presence of both a choice Bronsted acid and a choice organotrifluoroborate.
In most cases, 19F-NMR spectroscopy was employed to provide a rapid assessment of yields as well as tracking the fate of the RBF3 (in some cases the evolution of of free fluoride and of BF4 is observed). Following optimization, yields reported are isolated for n=2,3. We also found that the RBF3 must be used stoichiometrically and that it cannot be used as a catalyst in the presence of excess fluoride ion.
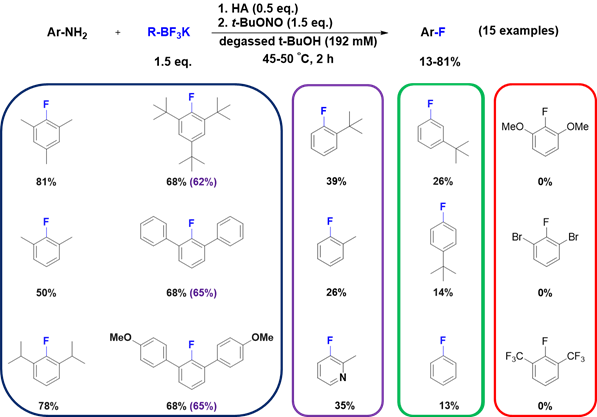
Results of this investigation led to an interesting reactivity trend shown below (Figure 4) whereby certain anilines gave exceptional yields while others gave lower and, in some cases, very poor yields. In most cases the phenol is produced as a side product; in cases where fluorination yields are low, the hydroxylation to give the corresponding phenol are elevated. Also of note, little if any reduction to the arene or cross-coupling to the trifluoroborate are observed.
Figure 4: substrate scope with various anilines that give the corresponding aryl-fluoride. Isolated yields are reported (n = 2 or 3).
Two notable trends emerge: 1) sterically hindered substituents at both ortho positions seem to favor high-yielding fluorination whereas a single ortho substituent reduces yields considerably and no ortho substituents further drop yields; 2) electron-withdrawing groups such as trifluoromethyl groups or bromine atoms also greatly retard fluorination. In these cases it was expected that the aniline would exhibit reduced reactivity towards diazotization. Hence, to address this concern, the diazonium salts of 2,6-disubstituted anilines were prepared and found to be refractory to productive fluorination. Interestingly, dimethoxy groups, which are inductively electron withdrawing through bonds but are considered electron-donating through resonance, also retarded fluorination. While similar trends have been observed by Zollinger et al. for dediazoniation, it is challenging to understand this trend in terms of standard carbocation stabilities since aryl-cations are not stabilized to extensively by ortho-substituents without invoking carbene-like resonance forms.
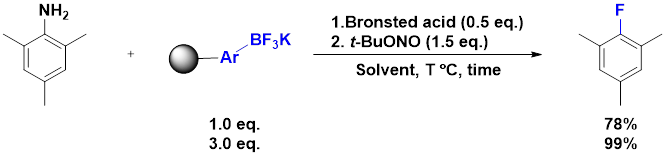
To explore the utility of organotrifluoroborates for flow-type reactions, we obtained a solid-supported orgnoboronic acid and converted it to the organotrifluoroborate. Various anilines treated in the presence of t-BuONO and a Bronsted acid and then flowed through a small column with mild heating. When using the trimethylaniline as the standard test substrate, yields were nearly quantitative, as indicated in Figure 5.
Figure 5: Flow-type fluorination under conditions where the organotrifluoroborate is supported on the solid-phase and the diazonium cation is introduced in simulated flow-type conditions.
In general, yields ranged from low to high depending on the diazonium salt used and followed the same trend as seen in Fig 4.
Summary of Support this Period:
The support provided has helped advance research in the area of fluoro-dediazoniation under mild conditions in inert solvents. This has several advantages including the use of low temperature and is made possible by the discovery that organotrifluoroborates can serve as competent fluoride donors in an updated Balz-Schiemann reaction. Ongoing efforts during the next reporting period will focus on using understanding the limitations in terms of substrate scope, addressing the mechanistic aspects that limit this substrate scope through the use of applications such as DFT calculation, isotope effects and kinetic assays, investigating several other carbon electrophiles, and addressing the possibility of intramolecular fluoride ion delivery.