Reports: ND756836-ND7: Controlled Polymerization Approaches to Polyelectrolytes with Resonance-Stabilized Phosphorus Cations
Kevin Noonan, PhD, Carnegie Mellon University
The incorporation of ionic groups into polymeric materials has been of enormous interest to both academia and industry. Electrostatic interactions of ions along a polymer chain alter the chemical and physical properties of a material relative to its neutral derivative. Polyelectrolyte properties are also dependent on the ion tethered to the polymer, as well as its counterion. While most stable cationic polymers are based on quaternized nitrogens, there is also significant interest in exploring 4-coordinate phosphonium-based materials since the larger atomic size and lower electronegativity alters charge distribution.
The tetraaminophosphoniums ([P(NR2)]4+) are derived from a class of organic superbases known as phosphazenes. This resonance-stabilized cation exhibits excellent thermal stability and small molecules have proven to be highly resistant to hydroxide anions for use as phase-transfer catalysts and for anion-delivery. There is tremendous difficulty incorporating these units into polymer materials, primarily due to synthetic challenges. In this proposal, we described our goals to prepare tetraaminophosphonium monomers for use in controlled radical polymerization to make well-defined polyelectrolytes. We planned to synthesize block copolymers and explore self-assembly of these structures to investigate how the cation affects stability and ion-transport. Finally, we noted our interest in more complex architectures bearing these units. Ultimately, we proposed to explore the fundamental properties of these tetrakis(dialkylamino)phosphonium materials, and their ability to participate in ion-conduction.
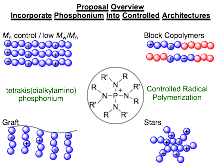
Goals (Figure 1)
1. Develop novel monomers compatible with controlled radical polymerization.
2. Synthesize block copolymers and complex architectures with a phosphonium monomer.
3. Investigate Properties.
Accomplishments
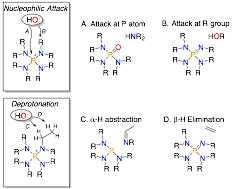
Over the past 12 months, we have two major accomplishments to report. First, as part of this research initiative, we wanted to obtain a better understanding of how chemically resistant tetraaminophosphoniums are under alkaline conditions. The data on decomposition of these cations is quite limited. We synthesized a family of eight different cations and explored degration in alcoholic solvents and in a biphasic reaction system. We discovered that in alcoholic solvents, b-elimination seems to be the most prevalent decomposition pathway while in two-phase systems (chlorobenzene and 50% NaOH/H2O), direct attack at phosphorus was the most relevant decomposition pathway (Figure 2). It should be stressed that very harsh conditions were employed to bring about degradation, which speaks to the impressive alkaline resistance of these cationic species. The goal of this study was to elucidate how these molecules break down to enable future design improvements. Additionally, the oxyanion driving decomposition in these studies is critical to rate and decomposition pathway for these cations. This paper was just accepted, which we will include in our updated citation list (10.1021/acs.organomet.7b00663).
This work has prompted us to compare and contrast other phosphoniums as well as ammonium cations. In particular, we are synthesizing aryl and alkyl phosphoniums to compare with their tetraamino counterparts, and additionally; we want to explore these in comparison to more classic alkyl ammoniums. We expect this will enhance our fundamental chemical understanding of cation stability to caustic anions such as hydroxide while also enabling us to appropriately select the most stable cations for tethering to polymer backbones.
Our second major accomplishment includes a two-step synthesis of phosphonium monomers for polymerization. We essentially can create a single reactive site on the phosphonium cation that is amenable to further substitution by deprotonation ([(N(H)R')P(NR2)3]PF6). We have used this strategy to build styrenic-based monomers as well as norbornene-based monomers. The advantage of the new route is that it can be accomplished directly from PCl5 and only a single step requires air-free conditions. With no oxidation step, this is indeed one of the simplest routes to make unsymmetric phosphonium monomers with R substituents that can be tailored for stability and an R' substituent that can be tailored for polymerization. We have begun to explore a variety of different polymerization strategies for these monomers. We have conducted some work with RAFT initiators to accomplish the initial stated goals but these routes have been more difficult than expected. We wanted to copolymerize the styrenic phosphonium monomer with butadiene and isoprene to produce block copolymers where one block would be glassy and the other elastomeric. This has been achievable but polymers with suitable mechanical properties to produce robust films have not been facile. We are continuing to explore this to obtain triblocks, but new initiators must be synthesized which has required more time.
The norbornene monomers that have been synthesized have also been evaluated in metathesis chemistry to build polyolefins and these materials are significantly more robust than styrenic systems. We will continue to explore copolymers bearing the phosphonium unit using both polymerization strategies. We are intending to publish the new synthetic route to the monomer as well as some copolymer data within 4 months.
Figure 1. Proposal Overview.
Figure 2. Decomposition pathways of [P(NR2)4]+ cations. ADDIN EN.REFLIST