Reports: ND1055315-ND10: Is the Road to Hydrodesulfurization Paved in Bronze?
Sarbajit Banerjee, PhD, Texas A&M University
Overview of Goals and Summary of Research Accomplishments: The primary focus of our ACS PRF grant is to (i) elucidate the distinctive electronic structure of the edges of MoS2 thought to be of critical importance for its catalytic activity; and (ii) develop intercalated MoS2 phases and map their catalytic activity towards hydrodesulfurization. Over the 2016—2017 reporting period, we have made substantial progress on both goals. Our specific research accomplishments are as follows:
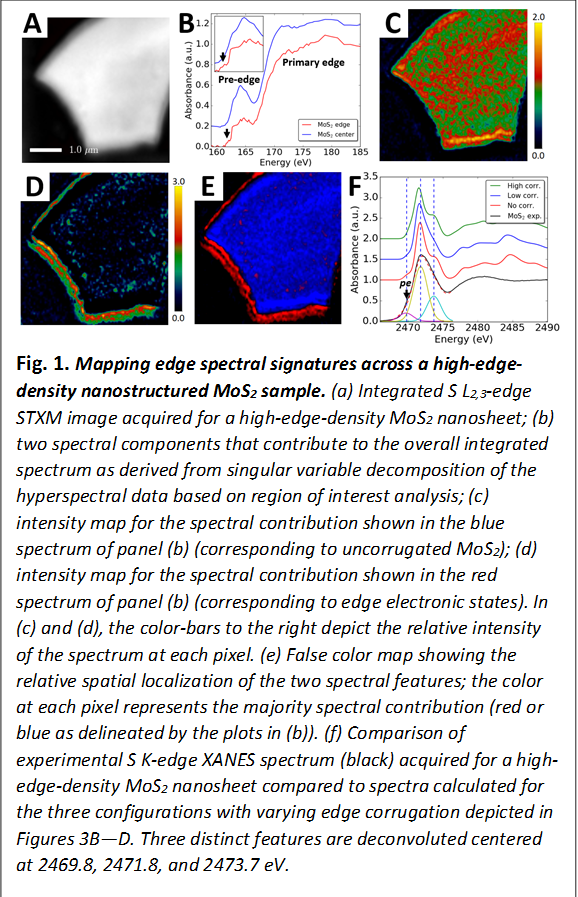
(i) Identification of Distinctive Edge Electronic States Correlated with Catalytic Activity: Using a combination of S L2,3- and S K-edge spectroscopy and scanning transmission X-ray microscopy imaging along with first principles density functional theory calculations, we have identified distinctive spectroscopic signatures from truncated edge electronic states and have directly correlated their appearance to catalytic activity. This work provides the first direct identification of the specific electronic states needed to mediate catalysis and provides a rational means of modulating edge reactivity.
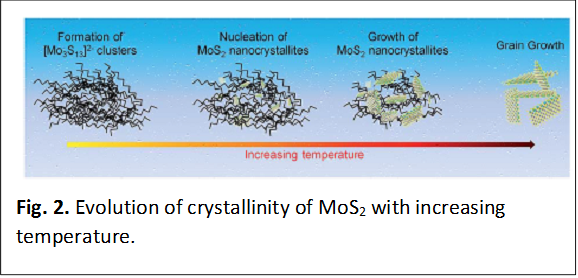
(ii) Mapping the Evolution of the Catalytic Activity of MoS2 Across its Amorphous to Crystalline Transition: We have mapped the catalytic performance of MoS2 catalysts as a function of the annealing temperature across their amorphous to crystalline phase transition. Studies of local structure indicate that with increasing annealing temperature, molecular precursors are initially cross-linked to form [Mo3S13]2- clusters characterized by both apical/bridging S22- and unsaturated/terminal S22- moieties, which in turn are consumed to nucleate ultra-thin crystalline MoS2 domains. With increase of the annealing temperature, these nuclei coalesce to form larger nanosheets. Annealing and the resulting amorphous to crystalline transition involves a trade-off between the number of available sites (which is decreased with increasing crystallite size) and the intrinsic activity of the sites (which is improved with increasing crystallinity). (Journal of Materials Chemistry A 2017, 5, 5129 – 5141.)
(iii) Modulation of the Reactivity of MoS2: We have examined novel approaches for enhancing the catalytic activity of MoS2 based on (a) interfacing with C60 molecules and (b) incorporation of Se dopants in S sites.
Mapping Edge Electronic Structure of MoS2: MoS2 is known to be an excellent catalyst for hydrodesulfurization of sulfur-rich hydrocarbon fuels. Specifically, the edges of MoS2 nanostructures are known to be the active catalytic sites. However, in the absence of precise elucidation of the geometric and electronic structure of the active catalytic sites, rational means of modulating edge reactivity remain to be developed. Over the 2016—2017 reporting period, we have demonstrated using first-principles calculations, X-ray absorption spectroscopy, as well as scanning transmission X-ray microscopy (STXM) imaging that edge corrugations yield distinctive spectroscopic signatures corresponding to increased localization of hybrid Mo 4d—S 3p states. Independent spectroscopic signatures of such edge states are identified at both the S L2,3 and S K-edges and the distinctive spatial localization of such states are evidenced in S L2,3-edge STXM imaging. STXM imaging at the S L2,3-edge indicates a pronounced abundance of such edge electronic states at the peripheries of high-edge density nanostructured MoS2 samples (Fig. 1); in contrast, such states are not observed for mechanically exfoliated MoS2 flakes with large crystalline domains and a low abundance of edge sites. The presence of edge corrugation serves to disrupt extended delocalization of Mo 4d states and yields low-energy hybrid states at the edge of the conduction band. The presence of such low-energy hybrid states at the edge of the conduction band is seen to correlate with substantially enhanced catalytic activity. The results provide clear elucidation of the edge electronic structure and provide a framework for its rational manipulation to enhance catalytic activity.
Mapping the Evolution of the Catalytic Activity of MoS2 Across its Amorphous to Crystalline Transition: The catalytic activity of MoS2 has been investigated across its amorphous to crystalline transition with increasing calcination temperature from 120 to 1000°C. Three distinct regimes are identified as depicted in Fig. 2: pre-nucleation, nucleation and crystal growth. Structural short-range ordering, associated with increasing amounts of the apical S22-/bridging S22-, is found at the pre-nucleation stage during low-temperature heating from 120 to 180°C in mostly amorphous molybdenum sulfdes comprising [Mo3S13]2- clusters and MoS3. Such a structural transformation brings about an increase in the density of active sites in the amorphous phase. However, the per-site activity of bridging S22- atoms is low compared with MoS2 nuclei and nanocrystallites. The nucleation of MoS2, progressing from 180 to 300°C in the amorphous phase, increases the overall catalytic activity. The anisotropic growth of MoS2 nanocrystallites from 300 to
500°C, results in deterioration of the overall catalytic activity as the active surface area is decreased; however, the per-site activity is gradually enhanced with increasing temperature. MoS2 crystallites embedded within an amorphous matrix exhibit the optimal combination of overall and per site activity.