Reports: UR654397-UR6: Spectroscopic Characterization of Acrolein in Its Lowest Triplet (n,pi*) State
Stephen Drucker, University of Wisconsin, Eau Claire
Acrolein (CH2=CH–CH=O) is the smallest alpha,beta-unsaturated carbonyl molecule and serves as a prototype for investigating the photochemical properties of larger analogs. Much of this photochemistry is mediated by triplet excited species, including the T1(n,π*) state. One goal of this project is to use jet-cooled cavity ringdown (CRD) spectroscopy to obtain experimental information about the structure and dynamics of acrolein in its T1(n,π*) state. During the past year of the grant, the PI and undergraduate collaborators completed modifications of our jet-cooling apparatus to convert the existing pinhole expansion into a planar-jet expansion. The planar expansion will afford a much longer absorption pathlength than the pinhole and will improve the signal-to-noise ratio in the planned CRD absorption studies of acrolein.
As part of this project, we will record the CRD spectra of deuterated isotopologues of acrolein. These measurements will provide rotational constants of the isotopomers in the T1(n,π*) excited state. These experimental rotational constants will allow us to determine the equilibrium geometry of the T1(n,π*) state of acrolein for comparison with high-level ab initio predictions.
A major component of this work is synthesis of the deuterated acrolein species. During the past year, the PI trained a junior-level undergraduate student to undertake the planned synthetic work. As part of her training, and as a prelude to the planned acrolein deuteration project, the student has successfully synthesized a related molecule, pyran-4-one (γ-pyrone). In ongoing work, we have been recording vibronically resolved CRD spectra of γ-pyrone in its T1(n,π*) excited state. The compound is commercially available, but it is costly. Our undergraduate collaborator has learned to prepare the key precursor, chelidonic acid, in large (100-g) quantities, using inexpensive starting materials including diethyl oxalate. She has proceeded to synthesize the target compound, γ-pyrone, obtaining samples with a higher purity than is available in the commercial product.
This training project has equipped our student with the necessary skills to carry out the acrolein deuterated work straightforwardly. At the same time, her successful preparation of γ-pyrone samples has propelled our spectroscopic work on this molecule and stimulated new avenues of investigation. The overall goals of the γ-pyrone project are similar to that of acrolein – to obtain experimental information about the structure and dynamics of the T1(n,π*) excited state. Results are being used as benchmarks for evaluating the accuracy of computational methods for treating electronic excited states.
The γ-pyrone molecule, like acrolein, has a conjugated enone chromophore. Both molecules have planar symmetry, and the T1(n,π*) excited states are straightforwardly classified within the C2v or Cs point group, respectively. However, displacement of out-of-plane vibrational coordinates lowers these symmetries (to C2 and C1, respectively), allowing the admixture of (π,π*) electronic character into the T1(n,π*) excited states. Under PRF support in the past year, we used a variety of excited-state computational methods to predict vibrational frequencies of γ-pyrone in its T1(n,π*) state. For out-of-plane vibrational modes (only), we found the predictions to be inconsistent from one method to another, and highly sensitive to the choice of basis set. We interpret this finding as an inadequacy in treating the multiconfigurational nature of the excited state. This outcome stimulated our interest in continuing an experimental project on γ-pyrone whose initial results we published in 2008. [L. Hoffelt, M. Springer, and S. Drucker, J. Chem. Phys. 128, 104312 (2008).]
In the previous work, we recorded a phosphorescence excitation spectrum of the T1(n,π*) ← S0 band system of γ-pyrone. From the spectrum, we determined fundamentals for several low-frequency vibrational modes in the T1(n,π*) state. However, the signal-to-noise ratio in the phosphorescence excitation spectrum was not high, which caused the vibronic assignments for a few of the observed bands to be tentative. Our very recent work has provided computational predictions of the frequencies, yet for the out-of-plane modes, the predictions of different methods do not agree with each other. This situation has prompted us to make improvements in our experimental approach for determining the frequencies. This will allow us to confirm or correct our previous vibronic assignments and obtain definitive experimental frequencies for the out-of-plane modes that can be compared to the (disparate) computational predictions.
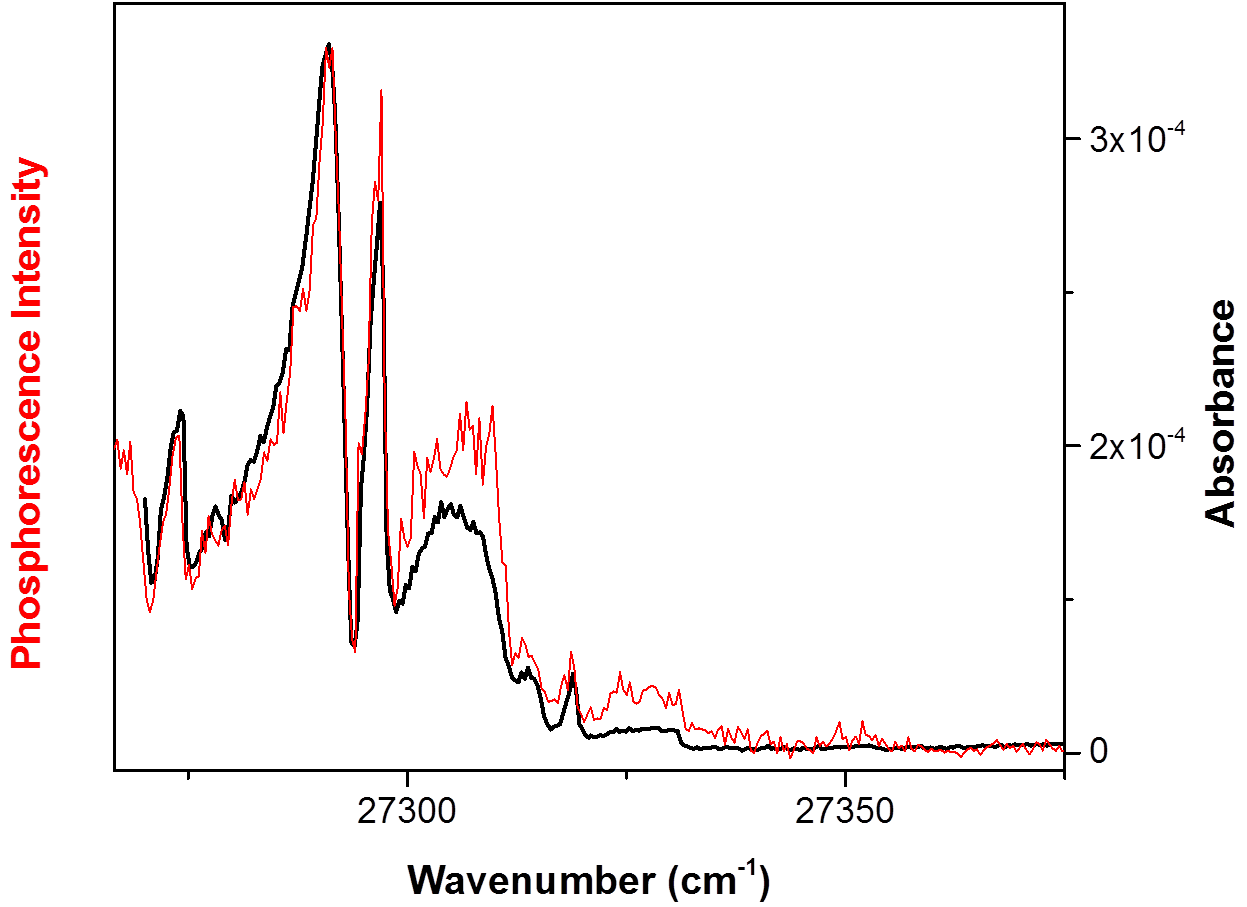
We conducted our most recent experimental work on γ-pyrone under PRF support during this past grant period. We are recording the T1(n,π*) ← S0 band system using CRD rather than phosphorescence excitation spectroscopy. In recent years we have improved the sensitivity and spectral coverage of our CRD detection system, so that now the technique affords a significantly better signal-to-noise ratio than phosphorescence excitation. The figure below shows our recently recorded room-temperature CRD spectrum (black) of γ-pyrone vapor in the region of the T1(n,π*) ← S0 origin band. Also shown is the phosphorescence excitation spectrum (red) we published previously. The signal-to-noise ratio is several times larger in the CRD spectrum. In particular, the modulation apparent in the CRD spectrum near 25305 cm-1 is reproducible rotational structure and not noise.
Currently we are recording the CRD spectrum of γ-pyrone in a higher-frequency region to secure vibronic assignments of the out-of-plane modes. We will also proceed with the preparation of deuterated isotopologues of acrolein, now that our student’s training in synthetic methods is complete. This will enable us to advance the planned jet-cooled studies of acrolein, using our newly incorporated slit nozzle source.