Reports: ND1054328-ND10: Novel Interrupted Polyacenes
Gordon W. Gribble, PhD, Dartmouth College
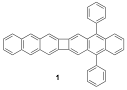
To address the oxidative instability of the parent linear acenes beyond pentacene, our project was to prepare "interrupted" polyacenes that will have the expected electronic properties of linear acenes but without the instability of the latter compounds. We have now synthesized the first example of a novel "interrupted" hexacene ring system, namely 5,16-diphenylcyclobuta[1,2-b:3,4-b']dianthracene (1).
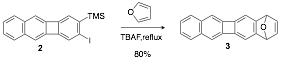
Most of today's synthetic routes to acenes employ arynes as Diels-Alder dienophiles, which are typically generated from o-(trimethylsilyl)aryltriflates or o-(trimethylsilyl)phenyliodonium triflate. However, as the chain grows, syntheses of the requisite aryne precursors require multiple steps. In our initial synthetic strategy to acene 1, the desired aryne precursors were not only formed in small amounts, but could not be isolated/separated from the reaction mixture. After extensive experimentation we found that o-haloaryl(trimethylsilyl) precursors were a most convenient aryne precursor and were also fairly soluble in most organic solvents. Thus, treatment of o-iodoaryl(trimethylsilyl) 2 with one equivalent of tetra-n-butyl ammonium fluoride (TBAF) in THF at reflux generated the expected aryne which was trapped by furan to give the expected adduct 3 in 80% yield.
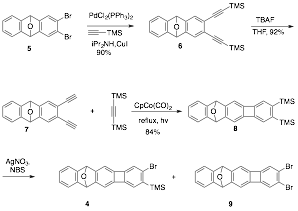
We extended this strategy to the use of o-bromoaryl(trimethylsilyl) 4, which was synthesized from 2,3-dibromo-9,10-dihydro-9,10-epoxyanthracene 5. Sonogoshira coupling reaction between 5 and trimethylsilylacetylene gave bis(trimethylsilylethynyl)-epoxyanthracene 6 in 90% yield. TBAF mediated desilylation gave diyne 7 in 92% yield and set the stage for a Vollhardt 2+2+2 cyclotrimerization with bis-trimethylacetylene (BTMSA) to furnish the epoxycyclobenzoanthracene 8 in 84% yield. Initial bromodesilylation (Br2/Py/CCl4) of 8 gave monobromo derivative 4 in 60% yield alongside 20% of dibromo derivative 9. Better yields of 4 were obtained by treating 8 with 1 equivalent of NBS and catalytic amount of AgNO3 in acetone at room temperature.
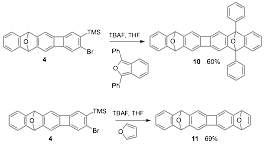
We next examined the aryne Diels-Alder cycloaddition reactions of 4. Thus, heating a mixture of 4, TBAF, and commercially available 1,3-diphenylisobenzofuran in THF gave 60% of endoxide 10. Using other fluoride sources and additives, e.g.., CsF or AgF in either THF or MeCN, we failed to meaningfully minimize protodesilylation. Similarly, a Diels-Alder cycloaddition of 4 with freshly distilled furan furnished 11 in 69% yield.
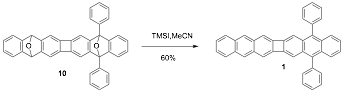
We first attempted the conversion of 10 to 1 under conditions that we had previously utilized for deoxygenative aromatization of structurally similar compounds. Thus, treatment of a solution 10 in THF with 10 equivalents iPrMgBr at reflux gave 40% of 1. To our delight, double deoxygenative aromatization with trimethylsilyl iodide (TMSI) proceeded well to give the desired product 1 in 60% yield. Acene 1 is a strongly fluorescent yellow solid that is bench stable under ambient conditions. We are now pursuing the electronic properties of 1 and the preparation of analogues.