Reports: ND556135-ND5: Studies of CO2 Photoreduction on Cu2O at the Single Molecule Level
Jay Gupta, PhD, Ohio State University
Aravind R. Asthagiri, PhD, Ohio State University
Motivation and Approach
Our goal is to study the fundamental surface science underlying the photo-electrochemical reduction of CO2 on nanostructured copper oxides, which have shown unique selectivity for methanol production. We combine scanning tunneling microscopy (STM) experiments and density functional theory (DFT) calculations to understand adsorption of CO2 on model surfaces. Experimentally, clean surfaces are prepared in an ultrahigh vacuum, cryogenic environment that enables us to image individual CO2 molecules and perform tunneling spectroscopy of molecular electronic states. The comparison of experiment and theory provides insight into adsorption sites, diffusion barriers and charge transfer, factors which determine the reactivity of CO2 on the surface.
Accomplishments
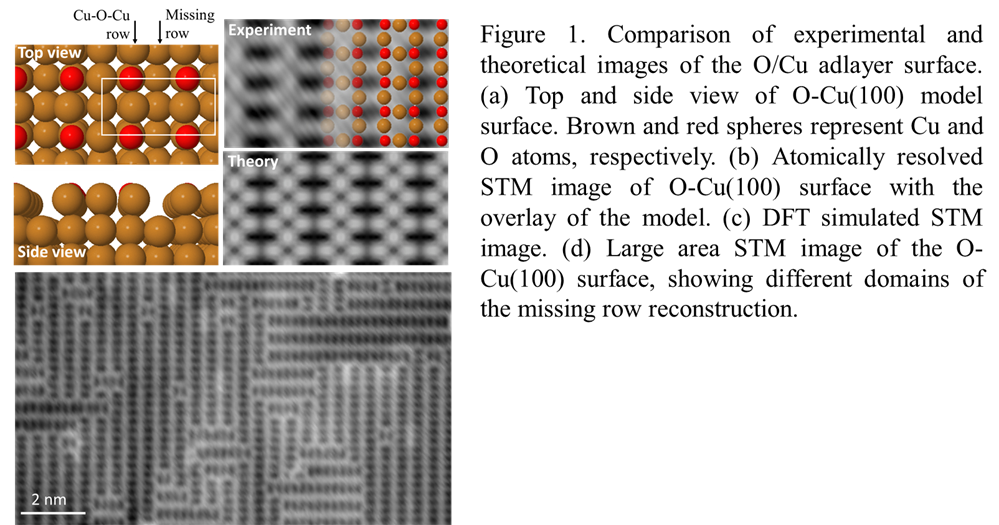
Our initial efforts have focused on studying CO2 molecules on an oxygen adlayer on Cu(100). We have successfully prepared the O-Cu(100) surface by thermal annealing a clean Cu(100) crystal at 310°C at PO2=1x10-6 mbar. The surface is then transferred into the STM, which is held at low temperature (5K) to limit surface contamination. The periodic slab model in Figure 1 indicates the expected missing-row reconstruction of the surface, where alternating O and Cu atoms are separated by a row of Cu atoms with vacant sites (referred as missing rows). Atomic-resolution STM images of the surface show a ladder-like contrast, with a unit cell of (3.59 Å x 7.19 Å), in good agreement with the calculated (2x1) unit cell of (3.64 Å x 7.28 Å). Comparison of the experimental image with the DFT-simulated image indicates that the ladder rungs locate the missing Cu rows, with pairs of oxygen atoms in the dark spaces between the rungs. A larger area STM image reveals two domain orientations with a 90° rotation, corresponding to nucleation along the [001] and the [010] directions of the Cu(100) surface. There is remarkably little surface contamination, evidenced by the complete lack of molecular adsorbates on the clean surface. This facilitates the identification and study of individual CO2 molecules on the surface.
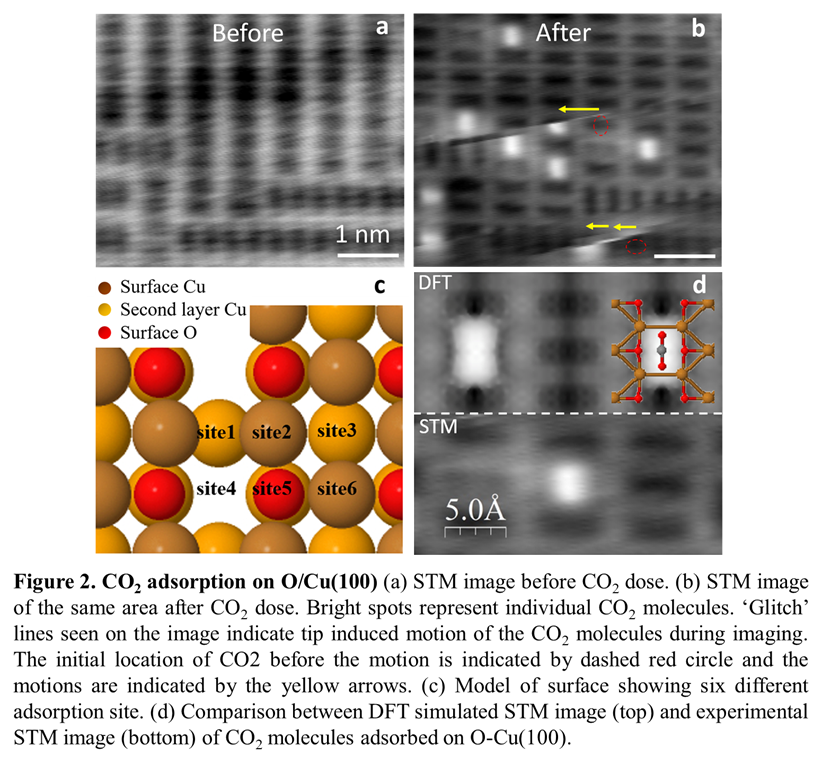
We deposited CO2 molecules in situ which allowed us to unambiguously identify CO2 and study the adsorption sites by comparing STM images of the same atomic-scale area before and after molecules were introduced into the chamber (Figure 2). We identified individual CO2 molecules as bright protrusions, elongated along the ladder-like structure of the O-Cu(100) surface. The molecules appear to sit in the gaps between the ladder rungs. DFT-simulated images confirm that the molecular axis is centered on, and parallel to the missing Cu row, with the C atom adsorbed between O atoms on the surface, and the O atoms in the CO2 closer to the Cu atoms in the rungs. DFT calculations considering 6 distinct sites indicate that this is the most stable adsorption site for CO2 on O-Cu(100), in agreement with the experimental observation that all of the molecules adsorb at this site. The DFT calculations also show that CO2 has a relatively low adsorption energy on the surface, and a similarly low diffusion barrier, which suggests that the molecules are weakly bound and could easily move on the surface. This is consistent with our experimental observations, where the STM tip perturbs individual CO2 molecules during scanning, evident as ‘glitches’ in the STM images. Tip-induced motion along and perpendicular to the ladder structure is observed, consistent with the similar calculated diffusion barriers for the two distinct directions on the surface.
Figure 3 compares tunneling spectroscopy of the CO2 molecules with the calculated density of molecular electronic states (DOS). Experimentally, we find that the CO2 exhibits relatively little distinct electronic structure compared to the surface, evidenced by the lack of pronounced peaks in the tunneling spectra. There is a slight increase in conductance near +/- 2V on the CO2 molecule, but no states in between. This is consistent with the calculated DOS, which shows a peak at -2.5 eV that is just off the range accessible experimentally and may explain the weak rise at -2 V, and a weak peak at +2.2 eV that may explain the weak rise at positive voltage. We calculated a HOMO-LUMO gap of 10.58 eV (HOMO: -5.21 eV, LUMO: 5.35 eV) for the adsorbed CO2 molecules, which is consistent with the lack of distinct states observed over the more limited range accessible experimentally.
Future Plans
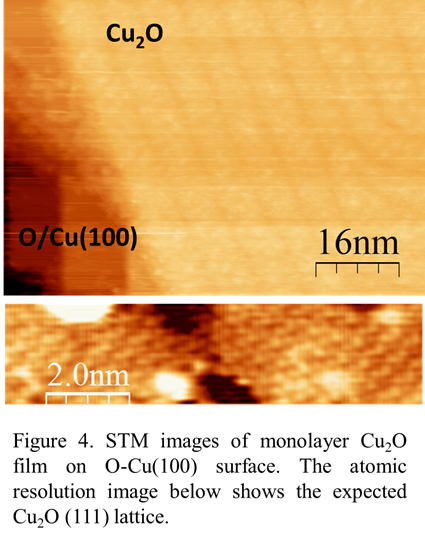
While preparing the CO2/O-Cu(100) results for publication, we are extending our work to study CO2 adsorbed on monolayer/multi-layer films of Cu2O, which more closely mimics the surfaces found in copper oxide catalysts. We will grow these films by depositing Cu onto the clean Cu(100) surface while maintaining an oxygen pressure of PO2=1x10-6 mbar. The advantage of this method is that we can controllably grow thin films of Cu2O with monolayer control, allowing us to study how the electronic structure of the films and CO2 adsorption depend on the thickness spanning single monolayers up to more bulk-like few nm-scale films. Figure 4 shows an STM image of our preliminary growth of a monolayer of Cu2O grown on Cu(100). Although the Cu(100) exhibits a square lattice, the Cu2O films exhibit a triangular lattice, with domains rotated by 120deg, indicating that the films are incommensurate with the surface. Strain with the surface may produce a periodic series of dark strips in the image. Atomic resolution images of the monolayer film indicate a lattice consistent with Cu2O(111). Now that we have demonstrated successful growth of the surface, we will deposit CO2 molecules and study molecular adsorption, while also developing theoretical models of the Cu2O(111)/Cu(100) surface structure.