Reports: UR453793-UR4: Mechanistic Studies of Radical and Radical Ion Intermediates in Oxidative Processes
H. J. Peter De Lijser, California State University, Fullerton
Research in our group focuses on the electron transfer chemistry of the carbon-nitrogen double bond. Of particular interest are oximes and oxime ethers because of their stability (compared to imines), their use as protection groups in organic synthesis, and the increasing use of these compounds in pharmaceuticals. Photooxidation or enzymatic oxidation may result in the formation of reactive species, such as radical ions or free radicals, which can cause damage to cells and tissue in biological systems. Our goal is to investigate the oxidative chemistry of oximes and related species and to obtain a complete understanding of the involvement of reactive intermediates in these processes.
In recent years we have shown oxidation of oximes leads to iminoxyl radicals as intermediates whereas oxidation of oxime ethers produces oxime ether radical cations. The different chemistry observed for each of these species is being explored for synthetic utilization. Of particular interest is to see whether we can perform intramolecular cyclization reactions to generate interesting heterocyclic structures. To do so we are exploring the intramolecular trapping of the radical and radical ion species with built-in alcohol groups, aromatic rings, alkynes, and alkenes.
Much of our foundational work in this particular area has been done using a series of o-arylbenzaldehyde oxime ethers (Figure 1) as well as a series of o-alkynylacetophenone and benzaldehyde oximes and oxime ethers (Figure 2).
Figure 1. Set of o-aryl benzaldehyde oxime ethers used for studies of intramolecular cyclization reactions of iminoxyl radicals and oxime ether radical cations.
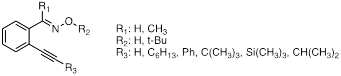
Figure 2. Set of o-alkynylaryl oximes and oxime ethers used for studies of intramolecular cyclization reactions of iminoxyl radicals and oxime ether radical cations.
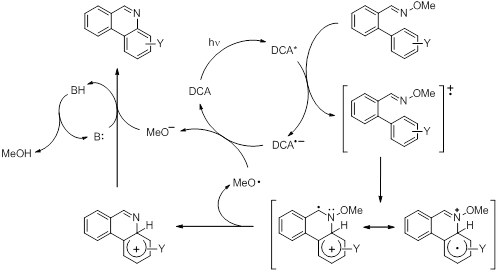
Both oximes and oxime ethers were studied but only oxime ethers were found to give satisfactory results in terms of cyclization, suggesting that the reactions proceed via the oxime ether radical cation. Under the conditions of the reaction, the photoinduced electron transfer (PET) process was found to be catalytic in nature and good yields and regioselectivity of phenanthridines could be obtained when using substituted aromatic rings (Scheme 1).
Scheme 1. Proposed mechanism for the formation of phenanthridines from the 2’-arylbenzaldehyde O-methyl oxime ether radical cation intermediate.
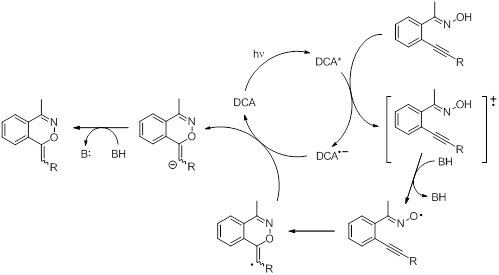
To learn about the alkyne moiety and its behavior as a radical trap or a nucleophile, a variety of different alkyne containing substrates (oximes and oxime ethers) were prepared and studied. Both photochemical and thermal oxidations were carried out and the reactions were followed by GC/MS and NMR. Cyclization is believed to occur in several cases but only for oximes, suggesting an iminoxyl radical intermediate. We have started a collaboration with a theoretical chemist (Dr. Andrew Petit) who computational chemistry to calculate and predict NMR spectra of the proposed cyclic structures. Comparison of the theoretical spectra to those of the experimentally observed spectra shows good agreement in some cases, whereas in others there is a clear discrepancy. Experimentally, we observed that the isolated product for the o-alkynylaryloxime with R3 = Ph was ustable and degraded over time. Initially we attributed this observation to a proposed product with an exocyclic double bond (exo-type cyclization (Scheme 2)). Computationally, the calculated NMR spectra were more consistent with the product being an N-oxide product (Scheme 3), which could explain the instability of the product. Currently we are identifying ways to trap the product to prove its structure.
Scheme 2. Proposed mechanism for the catalytic oxidative cyclization of o-alkynylaryl oximes.
Scheme 3. Proposed alternative mechanism for the catalytic oxidative cyclization of o-alkynylaryl oximes
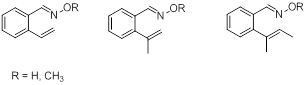
Because the alkyne and aromatic substrates discussed above seem to react differently, we have recently set out to prepare a set of alkene-containing substrates (Figure 3) to determine their reactivity.
Figure 3. Set of o-alkenylaryl oximes and oxime ether used for studies of intramolecular cyclization reactions of iminoxyl radicals and oxime ether radical cations.
All of the alkenylaryl oxime ethers react to give cyclic products although the yields vary significantly. The most promising results were obtained with the isopropenyl derivatives but interestingly both the oxime and the oxime ether give the identical result. We have made good progress in these reactions and by scaling up the reactions we were able to isolate the cyclic product and characterize it. We have purchased the predicted product (Scheme 4) to prove its structure. Although we do not yet have an explanation for the observation that both the oxime and the oxime ether react to give the same product, we hope that the computational work can aid our investigation and understanding of these reactions. The significant difference in reactivity between the vinyl and isopropenyl derivatives suggests that the additional methyl group may cause a substituent effect. To further investigate this, we have recently prepared the derivative with an additional methyl group present on the alkene group. The experimental (photochemical) work on this last derivative will begin soon. The expected product for this particular reaction is shown in Scheme 4.
Scheme 4. Proposed products for the catalytic oxidative cyclization of o-alkenylaryl oximes.
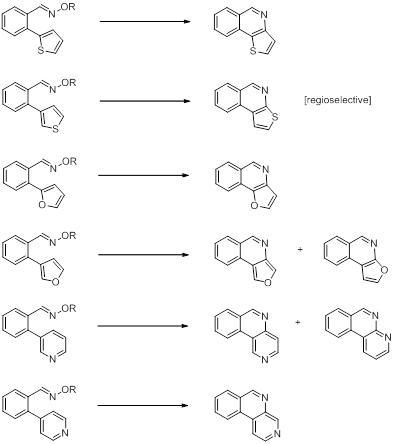
To further explore the potential of the cyclization reaction of o-arylbenzaldehyde oxime ethers, we have prepared a series of compounds with heteroaromatic substituents (Figure 5).
Figure 5. Set of oximes and oxime ethers with built-in heteroaromatic rings used for studies of intramolecular cyclization reactions of iminoxyl radicals and oxime ether radical cations.
Preliminary studies on the photooxidation reactions of these substrates suggests that cyclization can be observed with some of the oxime ethers. The best results were obtained with the thiophene derivatives whereas the furan and pyridine derivatives gave only small amounts of the expected cyclic product (Scheme 5). Interestingly, one of these reactions was found to be regioselective (based on NMR data). In addition, we have found a photosensitizer effect that needs to be further explored.
Scheme 5. Products from photooxidative cyclization reactions of oximes and oxime ethers with built-in heteroaromatic rings.
Current work is focusing on isolation and identification of the products. We also intend to explore the reactivity of the acetophenone derivatives to determine whether these reactions are influenced by steric effects.