Reports: UNI756629-UNI7: Development of New, Solvent and Catalyst-Free "Click-ene" Chemistry: One-Step, Green Chemistry Approach for the Low-Cost Synthesis of Petroleum-Based Building Blocks and Polymers for Industrial Applications
Santimukul Santra, PhD, Pittsburg State University
Development of one-step “click-ene” chemistry: Heat-assisted model reactions.
The main goal of this project is to establish new, one-step, solvent and catalyst free chemistry between alkyl azides and alkenes. We hypothesized that, the reaction between alkene and azide could be carried out at elevated temperatures or, in the presence of UV radiation or, may be under microwave conditions. This innovative green approach would be a powerful paradigm in the low-cost synthesis of petroleum-based building blocks, catalysts and polymeric materials. This reaction is named by the PI as “click-ene” chemistry.
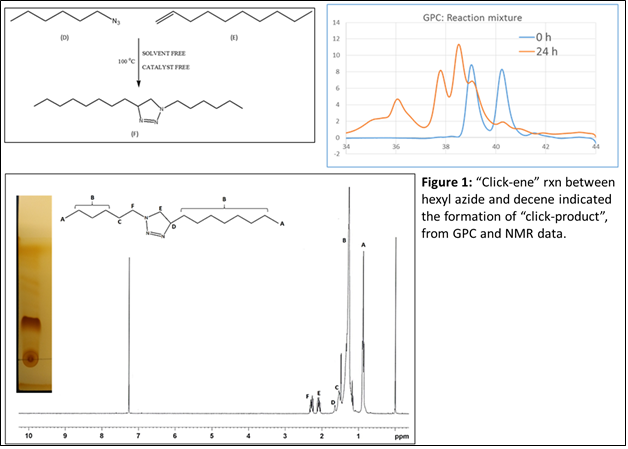
In the first year of the project, we have focused on few simple model reactions between azides and alkenes in the presence of heat. No other reagents including any solvent or catalyst are used. Towards this end, we have selected two simplest molecules, hexyl azide and decene (Figure 1) to validate our hypothesis and feasibility of “Click-ene” reaction. The reaction was performed at 90 oC and continued for 24 h. The expected “click-ene” product was found to be 90% pure before purification using flash column chromatography. The formation of triazole containing final product was confirmed by 1H NMR and GPC as shown in Figure 1.
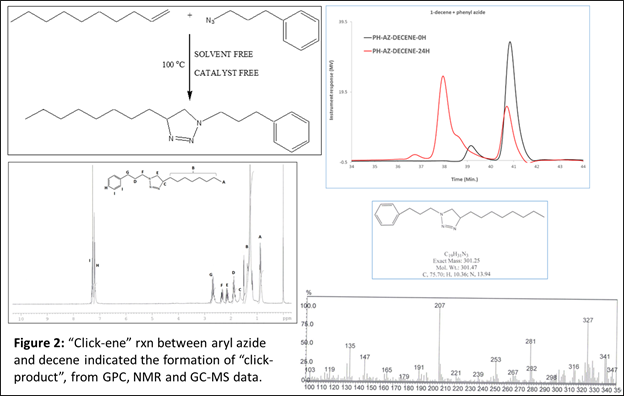
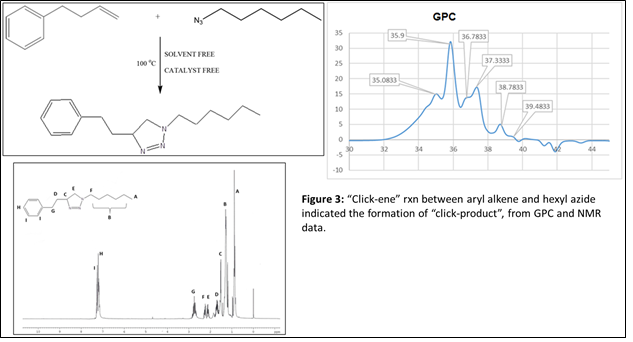
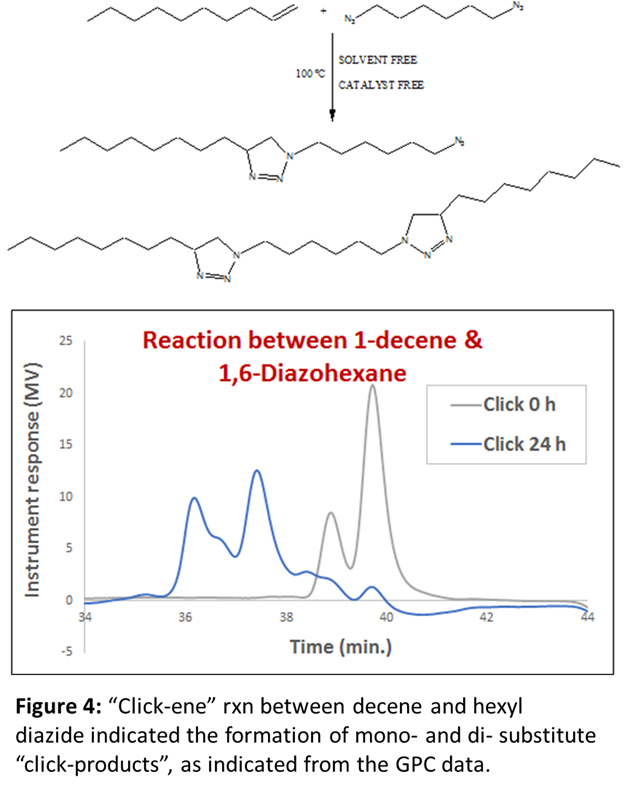
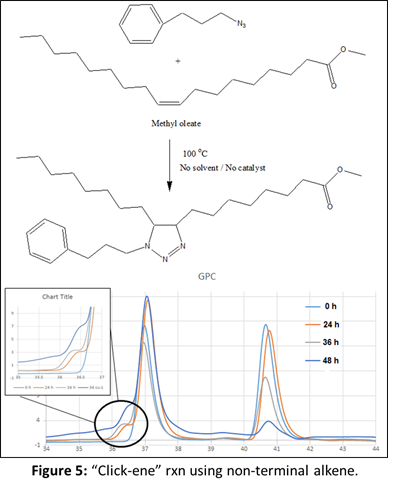
To further confirm the feasibility of this reaction under thermal condition, two more models reactions were designed where one of the starting material is composed of aromatic ring. We wanted to see, if there is any effect of aromatic double bonds in this “click-ene” chemistry. In one set of reaction, the azide component is composed of aromatic ring (Figure 2) and in another reaction the alkene reactant contents aromatic moiety (Figure 3). These additional two sets of reactions were performed under the same thermal conditions and the products were purified before characterizations. 1H NMR, GC-MS and GPC spectroscopic methods were used for the determination of the “click-ene” product. It was observed that the triazole ring containing final product obtained as major yields. In an additional set of reaction, a di-azide starting material is reacted thermally with an allyl precursor in the absence of any solvent or catalyst. As expected, a mono-substituted and a di-substituted products were obtained as indicated by GPC experiments (Figure 4). This indicated for the feasibility of the “click-ene” reaction in a specific stoichiometric ratio. The feasibility of this new chemistry with non-terminal alkene was also tested using methyl oleate and 3-phenyl propyl azide. The progress of the reaction was monitored using GPC (Figure 5) and found to be slow at 90 oC. The optimization of the reaction with non-terminal alkenes is in progress at various elevated temperatures.
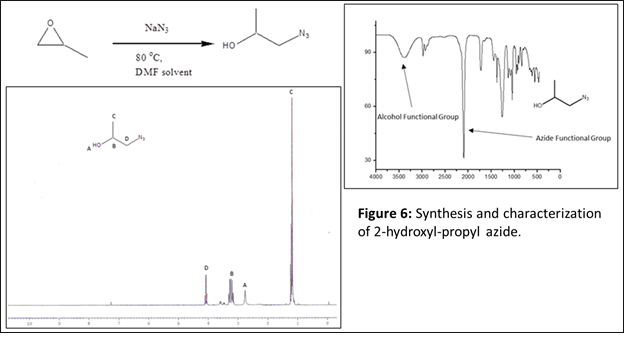
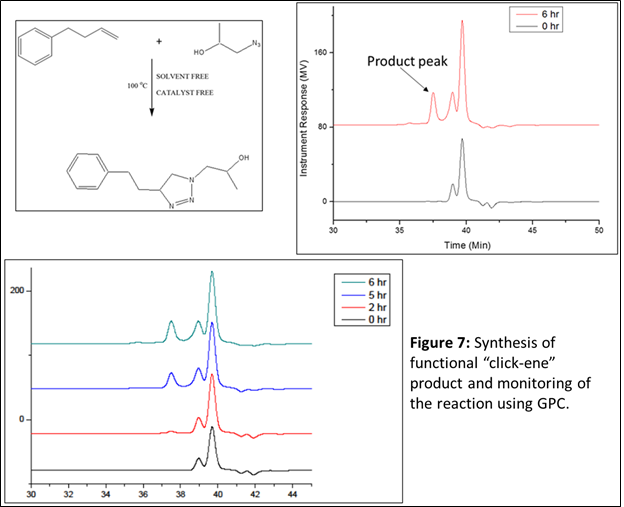
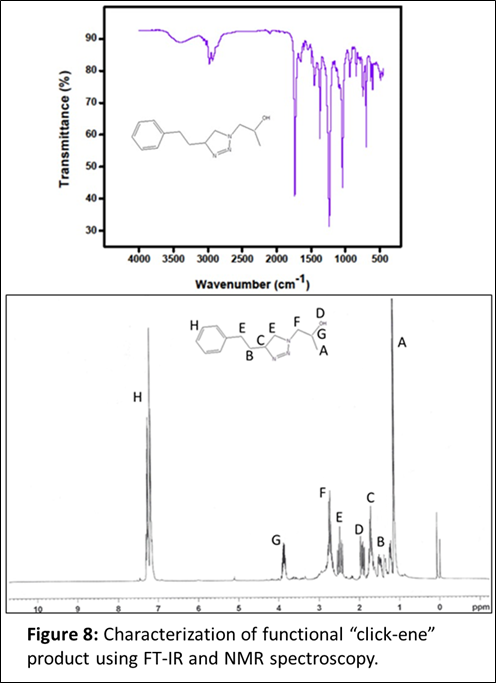
Next, we wanted to check for any possible cross-reactivity during the “click-ene” reaction in the presence of other nucleophilic groups along with the aromatic ring. We have designed a reaction using ‘2-hydroxy-propyl azide’ as the azide component and 4-phenyl butane as the alkene component. The 2-hydroxy-propyl azide was synthesized by reacting propylene oxide and sodium azide at 80 oC using DMF as a solvent. The purified azide component was characterized using FT-IR and NMR spectroscopy (Figure 6). As described earlier, the reaction between hydroxyl propyl azide and 4-phenyl-1-butene was performed without using catalyst and solvent (Figure 7). The reaction mixture was continued at 90 oC for 24 h. If successful, this product will have hydroxyl group in the structure, one-step ahead towards the formation of functional “click-ene” derivatives. The synthesized one-step hydroxylated product will be important for commercial applications. The functional “click-ene” product was purified quickly using flash column chromatographic technique. The resulting pure product was characterized using various spectroscopic techniques. The FT-IR experimental results demonstrated for the successful synthesis of the targeted functional “click-ene” product. The presence of a hydroxyl stretching band at 3400 cm-1, confirmed for the formation of the desired product (Figure 8). The hydroxyl functionalized “click-ene” product was further characterized by NMR spectroscopic method. This is a very important set of reaction, confirming for the synthesis of functional materials in one-step, without using any solvent or expensive catalyst.
Important application of heat-assisted “click-ene” reaction.
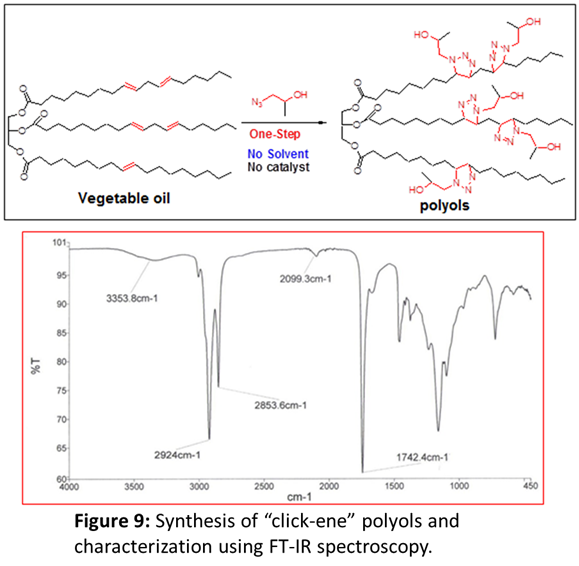
It will be interesting to find applications of this highly economic “click-ene” reaction. Towards this end, we realized that the industrial polyols are important synthons for the production of commercial polyurethane foams, cast materials, coatings, adhesives, sealants, elastomers and others. This important industrial polyols are generally synthesized in 2-3 steps (including epoxidations, hydroformylations, ozonolysis and hydrogenation) using toxic catalysts and therefore, laborious and expensive. Therefore, it will be great if one can synthesize this polyols in one-step. In the literature, vegetable oil-based polyols, for example, were synthesized in minimum of 2-3 steps using toxic chemicals, which ultimately, increases the market price of the materials. This prompted us to utilize “click-ene” chemistry in order to synthesize industrial polyols in one-step.
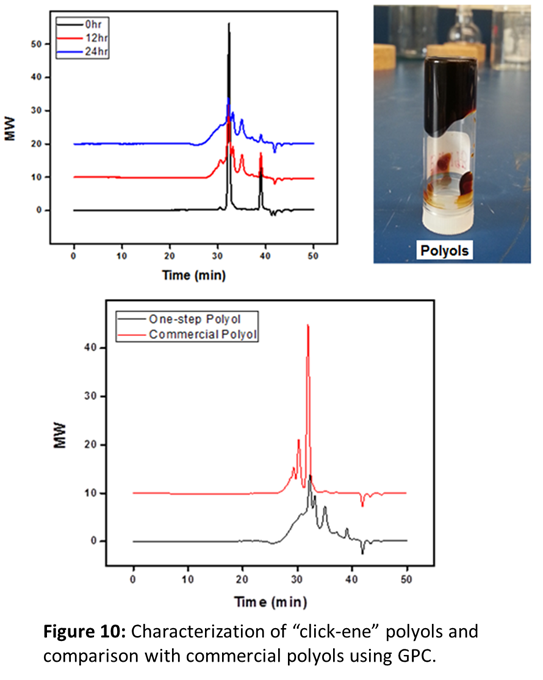
We planned to perform the “click-ene” chemistry with vegetable oil which contains multiple double bonds using previously synthesized 3-phenyl propyl azide. If successful, this would provide industrial polyols in one-step, reducing the usage of toxic chemicals and bringing it to the market in a very economical way. We hypothesized that in the similar fashion, propylene oxide azide would work with vegetable oil (e.g., soybean oil), to produce polyols in one-step without using any solvent or catalyst as shown in Figure 9. First, polyols sample after 24 h of reaction was analyzed using FT-IR. We have observed that the band for propylene oxide azide is disappeared, which indicated that the reaction has proceeded. In addition, appearance of a band at 3353 cm-1 confirmed for the synthesis of polyols. Next, the synthesized polyols was characterized by gel permeation chromatography, Mw = 1253, PDI = 1.62. However, after 24 h of reaction we have not observed any substantial change in the GPC, indicated that the reaction has completed within 24 h. It is interesting to notice that our one-step polyols yielded better (Figure 10, Black line curve) than that of commercial polyols from Cargill Inc. (Red line curve).
Next, we will establish this new reaction in the presence of UV or microwave and focus on new polymers.