Reports: ND353842-ND3: Studies of Magnetic Properties of Metalloporphyrins by Inelastic Neutron Scattering
Ziling (Ben) Xue, PhD, University of Tennessee
Over the past year, we have focused on the following: (a) Neutron scattering studies to other metal complexes including single-molecule magnets (SMMs); (b) Determination of the structure of Mn(TPP)F (H2TPP = meso-tetraphenylporphyrin) to understand why it is diamagnetic. In comparison, halide analogs Mn(TPP)X (X = Cl, Br, I) are paramagnetic.
Neutron scattering experiments were performed at Spallation Neutron Source (SNS) at Oak Ridge National Laboratory (ORNL) and Center for Neutron Research (NCNR) at National Institute of Standards and Technology (NIST) using Cold Neutron Chopper Spectrometer (CNCS), Vibrational Spectrometer (VISION), Single Crystal Diffractometer (TOPAZ), Backscattering Spectrometer (BASIS), and Disk Chopper Spectrometer (DCS). Ph.D. student Shelby Stavretis gave talks at the 253rd ACS National Meeting and the 2017 Neutron Scattering User Meeting at ORNL. Dr. Xue gave an invited talk at the ACS National Meeting. Two graduate students Zhiming Liu and Clay Mings have joined the group to work on spectroscopic studies of molecular magnetism. Several manuscript are being prepared for publication.
Our neutron scattering work has focused on determining the zero-field splitting (ZFS) of Co-based complexes with SMMs properties: Co(12-crown-4)2](I3)2, Co(PPh3)2X2 (X = I, Br, Cl), and Co(AsPh3)2I2. We have also studied Er[N(SiMe3)2]3 to determine its magnetic excitation. Quasielastic neutron scattering (QENS) of Co(acac)2(D2O)2 has been conducted to study methyl group rotation under magnetic field. Our studies of Mn(TPP)F were aimed at obtaining its crystal structure.
I. INS studies of Co complexes
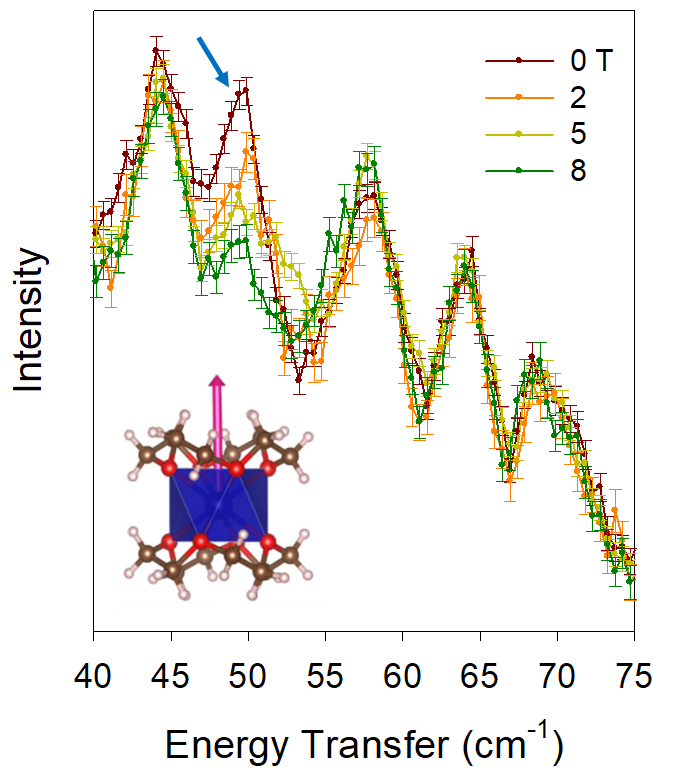
Co(12-Crown-4)2](I3)2. The magnetic anisotropic axis, determined at TOPAZ for 1, was used to align the crystals in the dirction of the magnetic field to observe Zeeman splitting of the ZFS peak. INS was conducted at CNCS with an external 8-T magnet, revealing the ZFS peak at 49 cm-1 (Figure 1). The ZFS peak shifts to higher energies as the field increases, showing that combining INS with a magnet is a definitive way to identify the ZFS peak. Previous magnetic susceptibility stuides determined ZFS to be ~75 cm-1 (JACS 2014, 136, 12213). It is clear fitting of the magnetic susceptibility data was not adaquate.
Figure 1. INS of 1 at variable magnetic fields. Blue arrow points out the ZFS peak. Structure of the cation of 1 is in the inset. Pink arrow represents the magnetic anisotropic axis.
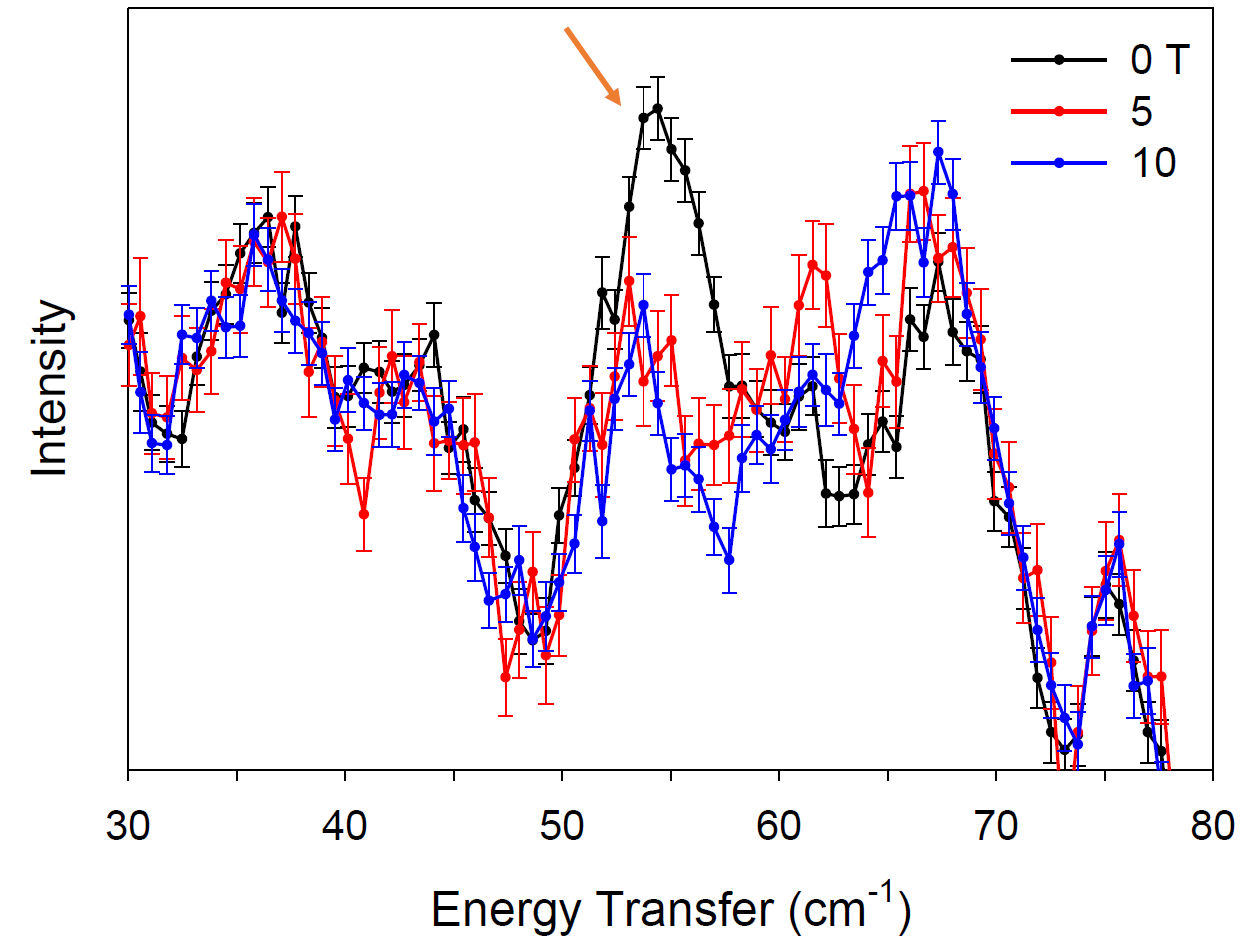
Co(PPh3)2X2 (X = I, Br, Cl) and Co(AsPh3)2I2. A recent study reported heavy and soft atoms enhance the magnetic anisotropy of SMMs (Chem. Comm. 2014, 50, 12266). Powder samples of these Co complexes were studied by INS at VISION and DCS with a representative spectrum of 2 (Figure 2). ZFS peak of 2 shifts to higher energies with increasing magnetic fields up to 10 T. Comparison of the new ZFS values from INS shows that fitting of the magnetic susceptibility data was not adequate to determine ZFS.
Table 1. ZFS values (cm-1) of Co(PPh3)2X2 (X = I, Br, Cl) and Co(AsPh3)2I2
SMM | Reported ZFS | ZFS determined by INS |
Co(PPh3)2Cl2 | -23.21 | │29.6│ |
Co(PPh3)2Br2 | -25.02 | │27.5│ |
Co(PPh3)2I2 | -73.83 | │27.3│ |
Co(AsPh3)2I2 | -149.43 | │53.2│ |
1. Chem. Comm. 2013, 49, 5289; JACS 2004, 126, 2148
2. Inorg. Chem. 2014, 53, 2367
3. Chem. Comm. 2014, 50, 12266
Figure 2. INS of 2 at variable magnetic fields. Orange arrow points out the ZFS peak.
II. INS studies of Er[N(SiMe3)2]3
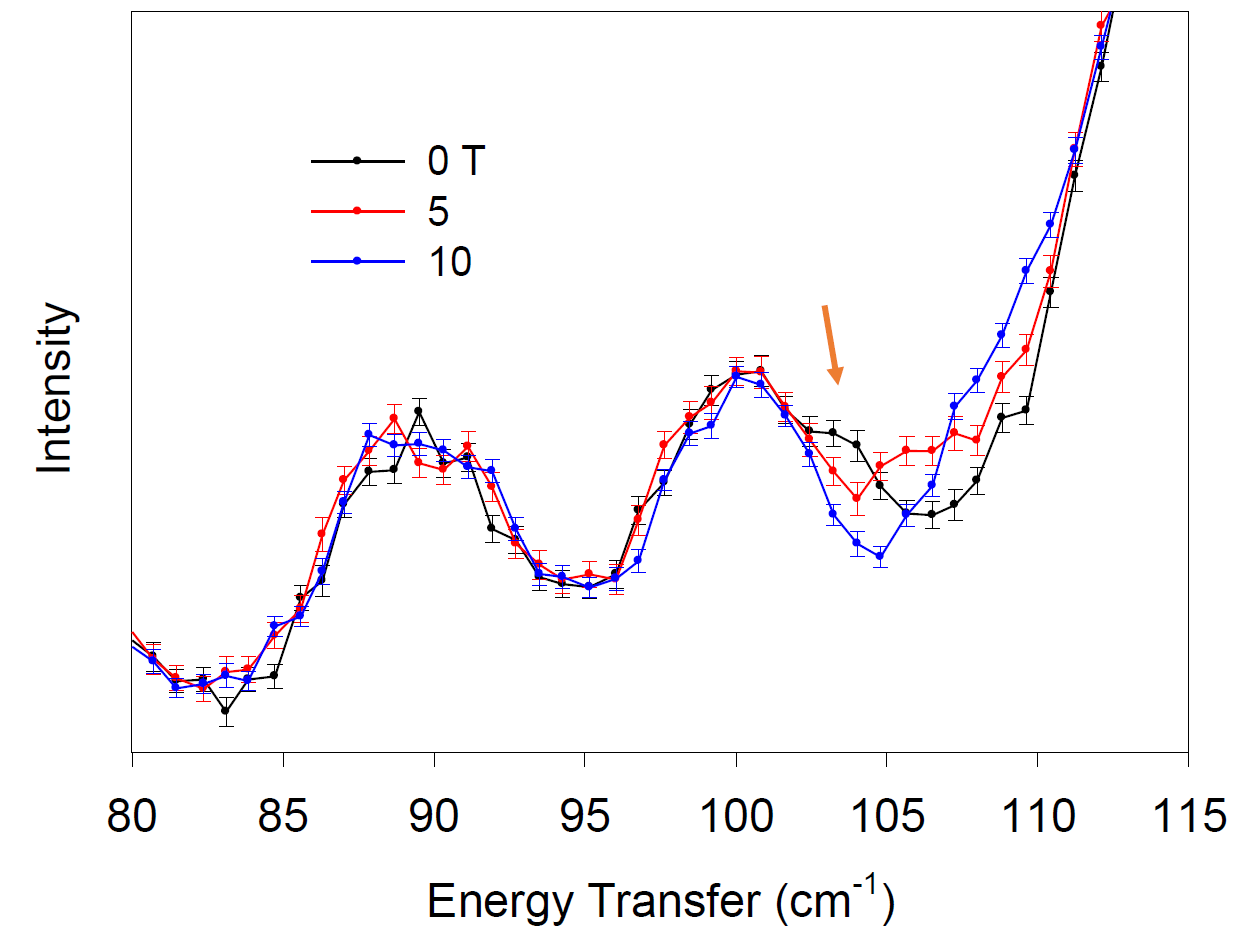
Er[N(SiMe3)2]3 (3) was the first equatorial, Er-based SMM (JACS 2014, 136, 4484). Previous studies determined the first excited state (mJ = -15/2 ® -13/2) at 85 cm-1 from fitting of ln(t) vs T-1 (t = relaxation time in the ac susceptibility measurement; T = temperature). Calculations revealed the first spin-obit coupled state to be 82 cm-1. Our INS with an external magnetic field directly determined this transition to be at 102 cm-1.
Figure 3. INS of 3 at variable magnetic fields. Orange arrow points out the ZFS peak.
III. QENS studies of Co(acac)2(D2O)2. Quasi-elastic neutron scattering (QENS) at BASIS has been paired with the 5 T magnet to study the dynamics of Co(acac)2(D2O)2 (4). In the temperature range studied, 80-100 K, the methyl group rotation is the dominant dynamical process observed. Surprisingly, it was found that the methyl rotation rate changes in response to the magnetic field. There are unpaired electrons stemming from the paramagnetic nature of the Co(II) ion. These electrons, however, are not localized on the Co(II) ion, but dispersed throughout the entire molecule. Since our work shows that the methyl groups respond to the magnetic field, this serves as an indication that some of the unpaired electron density is located on the methyl groups. The methyl rotation is actually slowed down by an increase in magnetic field possibly due to a stronger interaction between the dipole of the external field and that of the unpaired electron spins. To our knowledge, this is the first time the effect of magnetic field on methyl rotations has been observed with QENS.
II. Studies of porphyrin complex Mn(TPP)F
We published earlier (Inorg. Chem. 2014, 53, 1955) that Mn(TPP)Cl with four unpaired electrons (S = 2) has ZFS parameter D = -0.234 cm-1 through our INS work. Our later INS studies showed that both Mn(TPP)Br and Mn(TPP)I have ZFS parameters. However, Mn(TPP)F is diamagnetic. In addition, unlike its halide analogs which are molecular compounds and easily form single crystals, Mn(TPP)F has not formed single crystals in our repeated attempts. All we have been able to obtain are possibly polycrystalline samples. We have attempted to use powder X-ray diffraction to obtain the structure of Mn(TPP)F without success so far. The attempts include using the intense synchrotron-radiation light source research facility at ANL. We are currently making a new batch of Mn(TPP)F and its polycrystalline solid in order to determine its crystal structure. Once this part is completed, we plan to publish the paper reporting the structure and INS work on Mn(TPP)Br and Mn(TPP)I.