Reports: ND555270-ND5: Low Temperature Activation of Methane
Feng Jiao, University of Delaware
Recent progress has been made partially oxidizing methane at mild conditions (50 °C). An iron based zeolite catalyst has been used in the presence of hydrogen peroxide in this reaction. It has been proposed that the zeolite based catalyst activates hydrogen peroxide, forming a hydroperoxy species that is able to cleave the C-H bond. Major products of this chemical oxidation include methanol and acetic acid. In the past 12 months, we worked on new approaches based on this newly discovered low-temperature methane activation process. The key idea is to couple the low-temperature methane activation reaction with the low-temperature electrochemical hydrogen peroxide production. Electrochemical reduction of oxygen to hydrogen peroxide is desirable because it could be a decentralized process that occurs at moderate temperatures and pressures. In addition to this, the energy required could be provided by green, renewable sources, such as wind or solar power.
To investigate this low-temperature approach, we synthesized a series of N-doped carbon catalysts (details can be found in the previous report). To control nanoporosity in the N-doped carbon catalysts, we used the KIT-6 templates synthesized at three different hydrothermal temperatures, 80, 100, and 120°C and the resulting catalysts are labelled as NDC_80, NDC_100, and NDC_120, respectively. Elemental analysis showed that NDC_120 and NDC_100 had very similar C/N ratios of 3.35 and 3.73, respectively, while NDC_80 had a much larger C/N ratio of 7.71.
Electrochemical testing was carried out using cyclic voltammetry (CV), linear sweep voltammetry (LSV) and chronoamperometry, in both a RDE system and flow-reactor system. For the RDE system, 10 mg of NDC was sonicated in 1.99 mL of H2O, 0.5 mL 2-Propanol, 10 µL Nafion ionomer solution. 15 µL of the previous solution was dropcasted onto a 5 mm OD glassy carbon electrode. The solution was dried on a pre-polished GC electrode for 15min at 60°C. In order to evaluate the catalytic performance, a Princeton Applied Research potentiostat was used with a 3 electrode RDE setup. RPM was controlled by a MSRX control box. The counter electrode was a graphite rod. The electrolyte was 0.5M Na2SO4. Current was measured against a Ag/AgCl reference electrode and converted to the reversible hydrogen electrode (RHE) Scale.
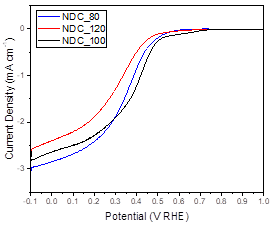
Figure 1 shows the LSV scans of NDC_80, NDC_100, and NDC_120. Onset potential is the lowest for NDC_100 followed by NDC_80 and NDC_120. The activity for NDC_120 is the poorest, as the current density is the smallest throughout the entire potential range tested. It is not clear from the scan if NDC_80 is better than NDC_100. Although the total absolute current is higher at potentials less than 0.3 V RHE for NDC_100, meaning that in general, more products were being created, it remains to be seen how the amount of peroxide being created at those potentials relates to the total amount of product. Although the onset potential is lower in NDC_80 than it is in NDC_100, the current is likely too small at these potentials to see any appreciable production of products. Therefore, it will be more important to look at more negative potentials for peroxide production.
Figure 1. LSV scans of NDC_80, NDC_100, and NDC_120.
At lower potentials (< 0.3 V RHE), the total current is largest for NDC_80. NDC_80 also had the largest electrochemical surface area (ECSA), followed by NDC_100 and NDC_120. As the ECSA increases, so does activity below 0.3 V RHE.
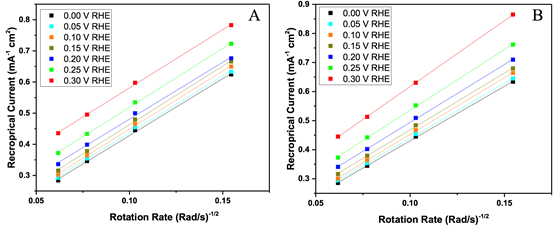
Figure 2. Koutecky-Levich plots at various potentials for (A) NDC_80 and (B) NDC_100.
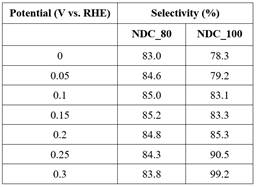
Koutecky-Levich plots for NDC-80 and NDC-100 are shown in Figure 2. Based on the Levich equation, a larger slope equates to a higher H2O2 selectivity. Across the 7 potentials shown, there is little difference in the selectivity of NDC_80. There is a clear difference between the selectivity at 0.3 V RHE and 0.0 V RHE. The selectivity actually decreases when moving towards higher overpotentials. The selectivity for each potential can be seen below in Table 1. The selectivity is very close to 84% H2O2 for all the potentials tested on NDC_80. This catalyst is surprisingly stable over the wide range of potentials tested. The other catalyst, NDC_100 has selectivities ranging from 78% to 99%. As expected based on the Levich plot, the highest selectivity is seen at the lower overpotential (0.3 V RHE). Due to mass transport limitations this trend is expected. As the overpotential increases, the rate of reaction increases. However, the rate of transport of reactants to the catalyst surface does not increase. These things combined lead to the over-reduction of the products, producing more H2O and less H2O2. The 99% selectivity seen in NDC_100 is among the highest seen in the literature. However, the significant drop off is disappointing. Many other NDC catalysts have stable stabilities in the low 90% for the same potential range as tested here. Based on these other catalysts 85% selectivity is disappointing. It is a good sign that it is at least stable, and should provide good partial current densities at higher overpotentials.
Table 1. Selectivity of NDC_80 and NDC_100 for hydrogen peroxide production.
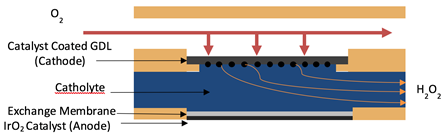
A flow cell is the best method to increase the rate of H2O2. It was clearly shown that the current increased in the RDE cell as the rotation speed increased, and therefore, the mass transport of reactants to the catalyst surface increases as well. An electrochemical flow cell was designed and constructed and the schematic is shown in Figure 3. The flow cell data are consistent with what are expected based on RDE testing and Koutecky-Levich plots. The selectivity increases as the potential nears the thermodynamic potential. Additionally, the current and concentration increase as the potential goes away from the thermodynamic potential. The concentration slightly decreases from 0 V RHE to -0.1 V RHE, but the decrease is very slight, under (10%).
Figure 3. A schematic of electrochemical flow cell design for hydrogen peroxide production through oxygen reduction.