Reports: UNI556993-UNI5: Molecular Interactions of Alkanethiols, Aryl Thiols, and Fullerenes in Multi-Component Self-Assembled Monolayers as Probed by Scanning Tunneling Microscopy
L. Gaby Avila-Bront, Ph.D., College of the Holy Cross
We chose to investigate the formation of a mixed monolayer composed of biphenyl-4-thiol (BPT) and octanethiol (OT). Both BPT and OT were carefully selected for this investigation due to their relevance in various industries.
BPT has been investigated in the literature for its applications in nanotechnology due to its enhanced mechanical stability as a monolayer and its role as a precursor in the creation of carbon nanomembranes. OT, on the other hand, is well established as an organothiol capable of forming SAMs that are stable over large temperature and pressure ranges. The goal of our investigation was to ascertain how a mixture of BPT and OT would assemble in ambient conditions, especially considering that our compounds are the same length and could interact along the entirety of their molecular axes.
We tackled this goal by focusing on two variables: 1. the concentration of the BPT solution used (either 0.2 mM or 100 mM), and 2. the sequence of deposition. We concluded that in no case did we observe structures similar to those found by Lussem et al., and that the final structure of the monolayer is significantly impacted by both of the variables tested. The fact that we did not observe arrays similar to those observed by Lussem et al. emphasizes the need to create a library of mixed monolayer systems, as slight differences in molecular composition lead to dramatic differences in monolayer structure.
XP spectra were collected at the Harvard University Center for Nanoscale Systems. Global characterization of the surfaces by XPS revealed shifts in the binding energy of the adsorbed carbon species. These shifts are especially pronounced for the samples in which OT is adsorbed before BPT, where the signal shifts closer to the position of the signal on a pure BPT monolayer.
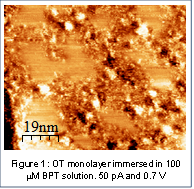
Figure 1 shows a representative image of BPT domains coexisting with OT domains. The particular region in Figure 1 shows disordered domains of what we presume to be BPT surrounding ordered domains of verified OT domains. Coexisting domains of BPT and OT were observed only when OT monolayers were exposed to the higher concentration of BPT, indicating that a threshold concentration has to be reached before the compounds interact. This result is of particular interest as it implies that multiple species will not mix on a surface unless they are present in certain amounts. This carries implications for developing films with blocking and passivating properties. Further application of this mixing behavior includes understanding the limit at which a species becomes a contaminant on a surface, which may change based on the characteristics of the components of the mixture.
Our study also discussed the displacement behavior of the molecules in the formation of the mixed monolayers. We believe that the displacement of molecules depends on the structure of the host monolayer and the presence of defect sites in this monolayer. Mixed monolayer systems studied in other groups have indicated different results, suggesting that the displacement can be system specific and underscoring the need to better understand the mechanism of mixing.
Students on this project: Danielle Fitzgerald, Emily Krisanda and Colleen Szypko ‘17
The next mixed monolayer that our group is investigating is a mixture of 2-naphthalenthiol (2NT) and octanethiol. Once again, the length of these molecules is on the same order of magnitude. We are interested in this mixture in order to directly compare it to our findings of the BPT and OT system previously discussed. 2NT lacks a bridge between the phenyl rings, which encourages p to p stacking and T-type interactions between the molecules. 2NT, therefore, should be able to form monolayers at ambient conditions that are more structured than BPT monolayers are at standard temperature and pressure. However, a previous study conducted by Jiang et al. demonstrated a complex heterogeneity of rotational domains in the surface. As a result of how the molecule tilts to form these rotational domains, the monolayer could also be stabilized by parallel-displaced p to p stacking interactions. Yet this 2006 paper by Jiang et al. represents the only characterization with molecular resolution of a monolayer of 2NT using scanning tunneling microscopy. We believe that the variability of the 2NT domains makes this molecule particularly difficult to characterize. It is entirely possible that adsorbing 2NT together with OT on a surface would lock its surface structure into one configuration over another. There is, of course, only one way to confirm or disprove this hypothesis and that is by creating a mixture of 2NT and OT as the first stepping stone to our library of mixed molecular monolayers.
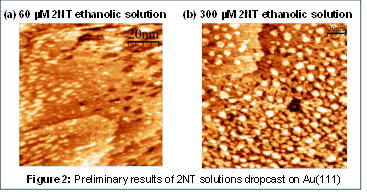
The images in Figure 2 show our preliminary results of 2NT deposited on Au(111)-on-mica. The 2NT was deposited by dropcasting ethanolic solutions of 2NT at varying concentrations onto a flame-annealed Au(111) surface. Figure 2(a) shows a representative image of a 60 mM ethanolic 2NT solution, while Figure 2(b) shows a representative image of a 300 mM ethanolic 2NT solution. On the whole, STM data of a monolayer of 2NT in ambient conditions reveal a surface covered in disordered “cloudiness” and bright features. We have assigned the bright features to either be clustered islands of the molecule adsorbed to the surface, or the protrusions associated with the formation of an aromatic organothiol molecule. An increase in presence of these features is correlated with an increase in the concentration of the solution of 2NT in which the surface is immersed..
Students on this project: Suhasini Aravinthan ’18, Keegan McCabe, ’18, and Hawar Haddadi, ’19