Reports: UNI255428-UNI2: The Formation of Salt Dome Cap Rock Calcites and Relationships with Sulfate Reduction
Sean J. Loyd, PhD, California State University, Fullerton
Introduction
Salt domes represent significant liquid petroleum and natural gas traps. Locally trapped hydrocarbons can experience degradation through microbially mediated processes largely supported by reactants sourced from the salt itself. As this hydrocarbon is degraded, authigenic carbonate minerals and elemental sulfur can form representing a hallmark "geobiologic" system. Supported by PRF, my lab uses geochemical techniques to more specifically identify the microbial processes involved in mineral formation.
Results, Implications and Ongoing Study
It is well established that hydrocarbon degradation occurs in proximity to salt domes in the Gulf Coast. Although speculated in the literature, the specific mechanisms facilitating degradation are poorly understood. Upon degradation, hydrocarbon carbon is oxidized by sulfate to produce dissolved inorganic carbon (DIC) and sulfide species in pore waters near salt domes. DIC reacts with dissolved calcium to produce calcium carbonate minerals and aqueous sulfides are oxidized to form extensive accumulations of elemental sulfur. Geochemical analyses of carbonates and elemental sulfur provide insight into 1) the hydrocarbon degradation mechanisms and 2) the nature of the locally trapped hydrocarbon.
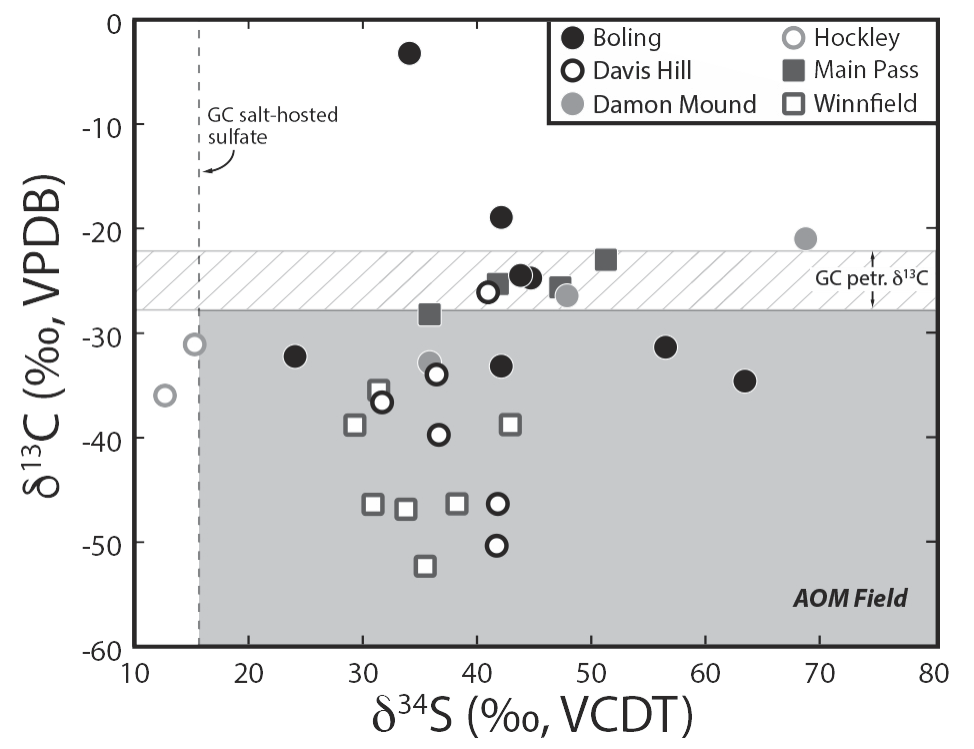
In order to address these issues, my lab has been conducting integrated carbon and sulfur geochemical analyses on samples derived from six Gulf Coast salt domes. Carbonate carbon isotope compositions (d13Ccarb) range from ~ –55 permil (VPDB) to near neutral, with most samples plotting below the liquid hydrocarbon (oil) end member (Figure 1). These d13Ccarb values suggest that these carbonates received a significant amount of carbon from the degradation of methane. Whereas these data provide insight into carbon sources (addressing #2 above), they do not reveal methane (or hydrocarbon in general) degradation mechanisms. Complimentary sulfur isotope analysis of trace sulfate incorporated into the carbonate lattice (so called carbonate-associated sulfate, CAS) allows the determination of sulfur-related degradation mechanisms and in particular sulfate reduction and/or sulfide oxidation processes. We find CAS sulfur isotope compositions (d34SCAS) that range from ~ +10 to ~ +70 permil (VCDT) (Figure 1). These values exceed the d34S values of local, salt-hosted anhydrite and gypsum that are thought to represent the original source of dissolved sulfate in these systems. Such high d34S values primarily result from closed-system sulfate reduction facilitated by microbes. When considered together, the carbon and sulfur isotope compositions suggest that the anaerobic oxidation of methane (AOM) acts to degrade methane, reduce sulfate and ultimately promote the precipitation of carbonates (and sulfur minerals) through the following reactions:
CH4 + SO42– = HXO3– + HS– + H2O (AOM)
Ca2+ + HXO3– = CaCO3 + H+ (calcite precipitation)
The discovery of AOM in salt dome systems is very intriguing, primarily as it is most widely reported from seafloor sediments associated with methane seeps, far removed from the subsurface depths at which salt domes occur. In addition, AOM is a microbial process, implying that salt domes host similar communities that live in the so-called "deep-biosphere".
Figure 1: Salt dome carbonate geochemical data. Samples that plot in the lower right quadrant indicate AOM. Those that plot outside of that quadrant may also form via AOM, however the data are non-distinct. All data aside from one point indicate closed-system sulfate reduction.
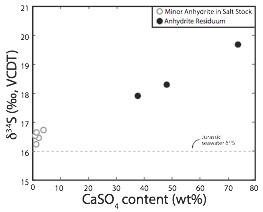
We have also been exploring formation mechanisms of anhydrite accumulations in salt dome settings. These anhydrites, interpreted as residual accumulations resulting from the preferential dissolution of halite in the salt stock, exhibit similar positive but less severe sulfur isotope enrichments (Figure 2). This indicates that the development of cap rock phases is more complicated than previously thought and that closed-system sulfate reduction plays a larger role than expected.
In order to better constrain the carbonate precipitation environment, we have also collected preliminary organic carbon isotope data (d13Corg) and clumped isotope data (D47). Similar to modern methane seep systems, d13Corg values of organic matter within salt dome carbonates are 13C-depleted with values extending down to ~ –50 permil. This likely reflects a significant methane carbon source although isotope fractionation associated with fixation pathways must also be considered when interpreting organic carbon isotope compositions. Preliminary clumped isotope compositions suggest carbonate precipitation temperatures ranging from ~15 to ~50 degrees Celsius, broadly consistent with shallow subsurface precipitation. However, recent research indicates that AOM may yield clumped isotope signatures inconsistent with precipitation temperatures, confounding interpretations.
Figure 2: Sulfur isotope composition of minor anhydrite within the salt stock (halite) and more pure anhydrite residuum. The relatively elevated d34S in the anhydrite residuum implies dissolution and reprecipitation from pore waters influenced by closed-system sulfate reduction.
Student Involvement
This research has funded five undergraduates and two gradate student working toward theses. The three undergraduate students include Kaelin Andelin, Connor Frederickson, Lucas Lu, Shawn Colby and Bayne Westrick-Snapp and these students have completed or are working toward undergraduate theses. Masters student Kylie Caesar has completed her thesis and we have submitted a manuscript to Nature Communications. Masters student John Hill has recently began his thesis exploring elemental sulfur formation mechanisms in salt dome cap rocks using multiple sulfur isotope techniques. All students have presented their research at multiple meetings (total of 15 abstracts). Kylie was awarded an oral presentation at the most recent Southern California Geobiology Symposium at Caltech, a particularly important achievement, as oral presentations are highly competitive at this venue. John Hill was awarded best graduate proposal at the Calstate Fullerton, Geology Research Day Symposium 2017.
Advancement of the PIs Career
This funding has provided an excellent platform onto which I have and will continue to build a promising and exciting research directive. As a young faculty member, I intend to use this directive as a cornerstone to reach tenure. Aside from new discovery and the opportunity to work with students, this research has promoted external collaboration. This work has allowed continued collaboration with a long time colleagues (Dr. Tim Lyons, UCR and Aradhna Tripati, UCLA) and development of a new collaboration (Dr. Rick Kyle, UT Austin and Dr. David Johnston, Harvard). I look forward to expanding this research (including beyond the PRF funding window), involving more students and collaborators, and to publishing results for dissemination to the broader scientific community.