Reports: UR456002-UR4: Fundamental Studies of 4,4'-Bipyridine Aryleneethynylenes
Nathan P. Bowling, PhD, University of Wisconsin, Stevens Point
The scientific objective of this project is the generation of novel aryleneethynylene compounds containing 4,4’-bipyridine subunits. Interest around these compounds centers around the potential to create novel coordination frameworks with transition metals or unique, dynamic conformers upon alkylation. The educational objective of this project is creating opportunities for undergraduate students to generate and study novel organic compounds that may have useful materials properties.
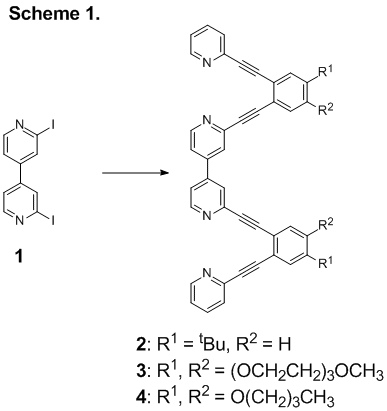
To this end, students have been working for the past year on generating novel 4,4’-bipyridine compounds. To date, the biggest challenge has been developing a reliable procedure for the synthesis of 2,2’-diiodo-4,4’-bipyridine (1, Scheme 1). Though we have been able to obtain enough to carry out the syntheses of several target compounds (described below), we have yet to land on an optimal procedure after several dozen tries.
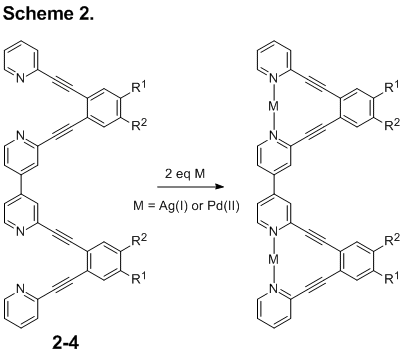
With the small quantities of 1 we have been able to generate, students have successfully synthesized several novel aryleneethynylene compounds. The simplest, synthetically most accessible, target was compound 2 containing tert-butyl groups for solubility. Unfortunately, though 2 itself is soluble enough in most solvents for analysis, the metal complexes of 2 crash out of all mixtures we tried. In order to make the arylene ethynylene compound soluble in more polar solvents that might be more conducive to study of metal complexes, students introduced triglyme solubilizing groups (3). With this structural variation, students were able to perform metal titrations in which Ag(I) and Pd(II) ions were separately introduced to solutions of 3. Chemical shift changes in the 1H NMR spectra and observed endpoints were consistent with binding of two equivalents of metal in solution.
The next step in this project is to obtain crystal structures that confirm the transition metal binding behavior of the novel aryleneethynylene system. Unfortunately, the solubilizing groups of 2 and 3 are not conducive to crystallization. The compound was instead generated with butoxy solubilizing group (4, Scheme 1). This sample is currently with our crystallography collaborator, Eric Bosch, at Missouri State University.
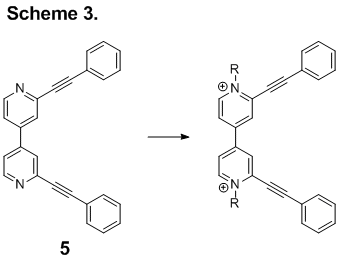
A second component of this project is to create novel 4,4’-bipyridine-based arylene systems that can be alkylated at the N-position. These viologen compounds are expected to have interesting electronic and magnetic properties. Functionalization with alkynes should alter the electronic properties and provide an avenue for conformation control. As with the project described above, our efforts have been slowed by our inability to reliably generate compound 1. With the small quantities of 1 that have been generated to date, students have synthesized phenyl acetylene derivative 5 (Scheme 3). Alkylation has been a bit problematic as the N,N-dimethylated version of 5 has limited solubility in every solvent we have tried. Attempts at other alkylations have led to poor yields of product. With the small amounts of N,N-dimethylated 5, students have been able to perform some initial cyclic voltammetry tests in DMF. While the sample studied did not have the two reversible oxidation-reduction potentials expected for a viologen, there could be several explanations, including aggregation or incomplete methylation. Currently, attempts to regenerate 5 are underway.