Reports: DNI956052-DNI9: A Fundamental Study on Non-Oxidative Methane Activation and Oligomerization Reactions using Bi-Metallic Catalysts to form Higher Hydrocarbons and Graphene
Hema Ramsurn, PhD, University of Tulsa
In order to understand the role of molybdenum during methane dehydroaromatization (MDA), Mo was loaded on an inert support, silica, by two different methods namely incipient wetness impregnation and sol gel method by co-precipitation. Molybdenum was also loaded on HZSM-5 (acidic zeolite support, commonly used for MDA) by incipient wetness impregnation (IWI) method. The prepared catalysts were characterized using a number of techniques, as described below.
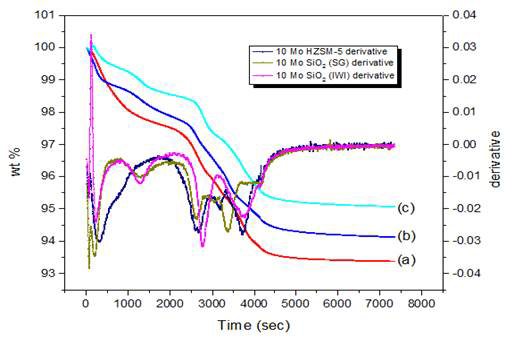
The thermogravimetric analysis (TGA) was used to quantify the amount of molybdenum present in the catalyst sample based on the decrease in weight percent during hydrogen reduction of the catalyst. Figure 1 shows the TGA curves of the catalyst samples with different catalyst supports. The loss in weight up to 200 °C corresponds to the loss of water. At around 500 °C, reduction of MoO3 begins with MoO3 being completely reduced to molybdenum at around 680 °C. Neglecting the weight loss up to 200 °C, there was a weight loss of about 4.24 % in case of Mo/SiO2 prepared by IWI, 4.59 % in case of Mo/SiO2 prepared by sol gel and 4.82 % in case of Mo/HZSM-5 which corresponds to 8.56 %, 9.27 and 9.73 % of molybdenum loading respectively, where the expected loading was 10 %. The decrease in molybdenum wt% might be due to its loss in the form of MoO3 during calcination at 500 °C.
Figure 1: TGA curves for reduction of (a)10-Mo/SiO2 (IWI), (b)10-Mo/SiO2 (SG), and (c)10-Mo/HZSM-5 under H2
The catalytic reactions were performed using a recirculating batch reactor system as shown in Figure 2. The products from the reactor are analyzed using an HP 5890 series II gas chromatograph with a flame ionization detector. From the catalytic reaction studies, it has been found that methane at 750 °C, in a blank reactor or in the presence of pure SiO2 or pure HZSM-5, gave negligible conversion. But in the presence of Mo/SiO2, ethylene, ethane and benzene were formed. Thus, molybdenum species are necessary for methane activation.
Figure 2: Schematic diagram of catalytic reactor system
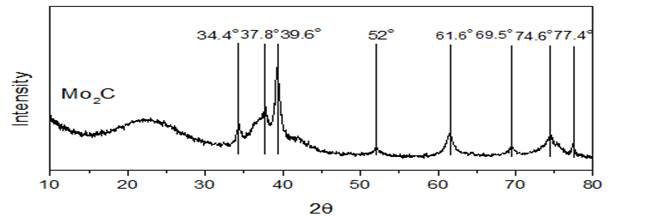
The used catalysts were examined using XRD (X-ray diffraction) to understand the nature of molybdenum sites that help in methane activation. The XRD pattern (Figure 3) of used catalysts (subjected to a mixture of 25% CH4/Ar at 750 °C) shows that the molybdenum species have been converted into molybdenum carbide sites which act as the primary sites for methane activation.
Figure 3: XRD pattern of used catalyst (10 wt % Mo/SiO2 prepared by sol gel method) for methane activation with reaction time of 130 mins.
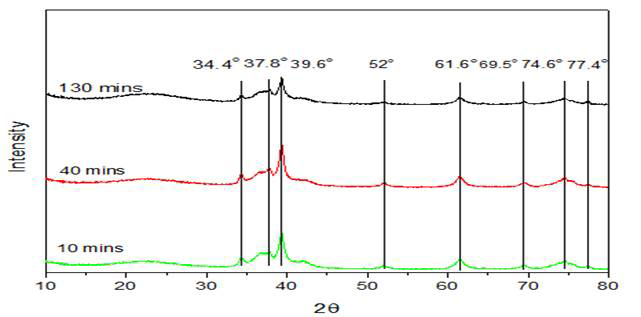
Figure 4 shows the nature of used catalyst at different reaction time. It was found that these active Mo2C sites started forming within the first 10 minutes of the reaction.
Figure 4: XRD pattern of used catalyst (10 wt % Mo/SiO2 prepared by sol gel method) for methane activation at different reaction times
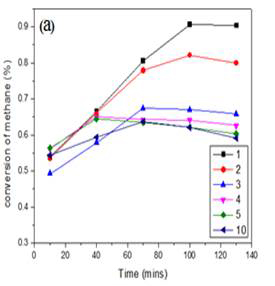
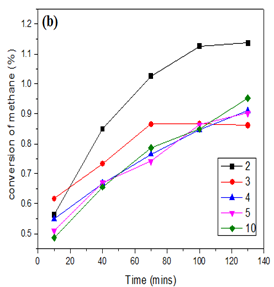
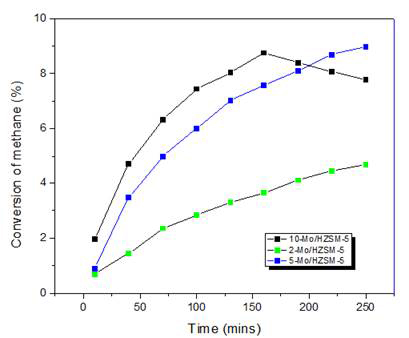
Thus, knowing that molybdenum species are necessary for methane activation, the performance of two different catalysts (Mo/SiO2 and Mo/HZSM-5) were compared for further understanding of the reaction mechanism. Maximum conversion of about 1.2 % at 750 °C was achieved using Mo/SiO2 prepared by co-precipitation method. Figure 5 shows the conversion of methane at 750 °C by Mo/SiO2 catalysts of different molybdenum loadings. When methane was activated over molybdenum loaded on acidic zeolite catalyst, the conversion was close to 9 % at 750 °C. Figure 6 shows the conversion of methane at 750 °C by Mo/HZSM-5 catalysts of different molybdenum loadings. At high loadings (10 wt %) as the time on stream increases, higher molecular hydrocarbons might be formed which might get stuck to the reactor walls and were not detected by the GC-FID detector. Thus, the conversion seems to decrease as the reaction time increases. Major products formed by methane conversion were benzene and ethylene with trace amounts of ethane, toluene and xylenes.
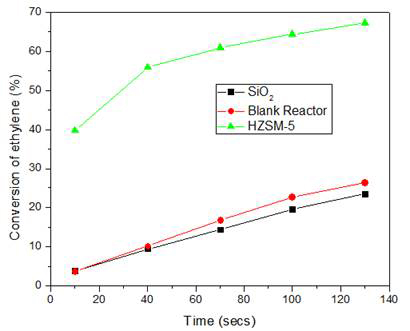
High purity ethylene was reacted with pure SiO2, HZSM-5 and on a blank reactor at 750 °C. Interestingly, even in the absence of molybdenum species ethylene was converted to higher hydrocarbons. In a blank reactor and using the inert silica support the conversion was close to 26% and 24% respectively with 27% benzene selectivity but when HZSM-5 was used, the conversion was close to 67% with 51% benzene selectivity. Figure 7 shows the conversion of ethylene by pure SiO2, HZSM-5 and blank reactor at 750 °C. Even in a blank reactor, ethylene was converted to aromatics at 750 °C which confirms that no active sites are required for ethylene oligomerization at high temperatures. But the acidic zeolite sites highly enhance the ethylene oligomerization reaction and selectivity of desired product. Thus, ethylene was the intermediate product formed during the activation of methane by active molybdenum carbide sites. Once this intermediate is formed, aromatics are formed by the oligomerization reaction.
Figure 5: Conversion of methane over MoO3/SiO2 catalysts with different Mo loadings prepared by (a)incipient wetness impregnation (b)sol gel method at 750 °C.
Figure 6: Conversion of methane at 750 °C over MoO3/HZSM-5 catalysts with different Mo loadings prepared by incipient wetness impregnation.
Figure 7: Conversion of ethylene at 750 °C over pure SiO2, HZSM-5 and on a blank reactor.
This part of research concludes that, molybdenum species in the form of molybdenum carbides helps in methane activation and in ethylene formation. They however do not play any role in the aromatization reactions. Ethylene can undergo oligomerization at 750 °C but using acidic zeolite as a catalytic support greatly enhances oligomerization and desired product selectivity.
This proposal has helped the PI to explore a new avenue of research and explore fundamental understanding of the mechanisms of catalytic methane-to-liquid reactions. Together with her student, she is preparing the first manuscript which should be submitted by the end of this year. The student, Vaidheeshwar Ramasubramanian, has learnt to build a set-up, prepare catalysts, run gas-to-liquid experiments and has also gained sufficient training and knowledge in working and troubleshooting instruments like ICP-MS, GC, SEM and XRD.