Reports: DNI156371-DNI1: Early/Late Heterobimetallic Complexes for Catalytic Alkene and Alkyne Functionalization
David John Michaelis, PhD, Brigham Young University
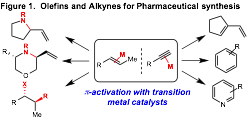
Simple, petroleum-derived olefins and alkynes are important building blocks for synthesis because of the myriad structures accessible via their functionalization. Transition metal-catalyzed reactions of olefins and alkynes are particularly important because a late transition metal can serve as a p-acid to activate them towards nucleophilic additions. The objective of this proposal is to develop heterobimetallic Ti–M catalysts capable of novel reactivity in C–N and C–C bond forming reactions via electrophilic olefin and alkyne activation (Figure 1).
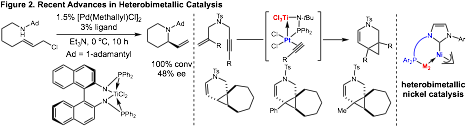
During the first 17 months of this project, we have focused on three different strategies to utilize heterobimetallic catalysts in synthesis. First, we have synthesized and tested a variety of chiral titanium ligands for enantioselective allylic amination reactions with hindered amine nucleophiles. Second, we have used titanium-containing ligands to assemble platinum-titanium catalysts in situ and facilitate fast cycloisomerization reactions (Figure 2). Third, we have developed N-heterocyclic carbene ligands (NHC) containing pendant coordinative groups for heterobimetallic nickel catalysis. Specific details of each of these pursuits are described below.
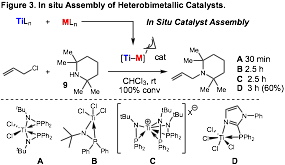
Enantioselective Bimetallic Catalysis: Recent efforts in the field of heterobimetallic catalysis have demonstrated that metal-metal interactions can facilitate new reactivity. What has not been reported, however, is the design and use of chiral heterobimetallic catalysts for enantioselective catalysis. One aim of our research is to develop chiral titanium-containing ligands that assemble heterobimetallic complexes in situ to provide highly active and selective catalysts for organic transformations with alkenes and alkynes. Previously, we have demonstrated that a variety of titanium containing ligands assemble bimetallic Pd-Ti catalysts in situ and enable fast allylic amination catalysis with hindered amine nucleophiles (Figure 3). Importantly, this allylic amination with hindered amines does not proceed at room temperature with any phosphine ligands that we have screened to date.
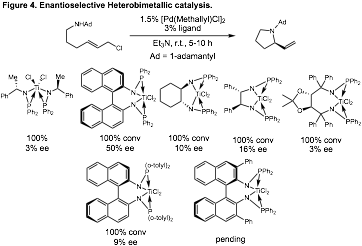
We synthesized a variety of chiral bisphosphine titanium ligands for use in enantioselective reactions. We then screened these ligands in allylic amination reactions that generate piperidine products (Figure 4). We were delighted to find that with our binaphthyl-derived ligand we could obtain enantioselectivities up to 48% ee. We have begun to optimize the structure of this binaphthyl ligand to improve this modest enantioselectivity. In addition, we have initiated computation studies with the Ess laboratory to calculate the enantioselectivity of different ligand scaffolds. These efforts are guiding our synthetic efforts in the design of ligands for enantioselective catalysis.
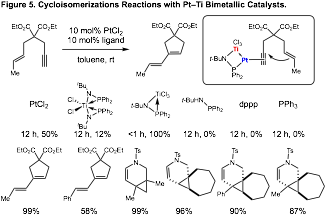
Heterobimetallic Catalysis with Platinum: Additional efforts in our laboratory have focused on applying our titanium ligands in reactions with metals other than palladium. Our initial applications have focused on platinum catalysis, where formation of a Pt–Ti interaction could accelerate catalysis in cycloisomerization reactions. In these studies, we demonstrated that while bisphosphine titanium ligands did not enable catalysis, the use of monophosphine titanium ligands generated active catalysts that enable reaction at room temperature (Figure 5). We believe that the monophosphine complex was active because it contained open coordination sites that could bind the alkyne substrate. Importantly, all phosphines ligands that lacked a titanium atom shut down reactivity completely, demonstrating the importance of the titanium to improve catalysis. Under our standard conditions, a variety of cycloisomerization reactions proceed in high yield.
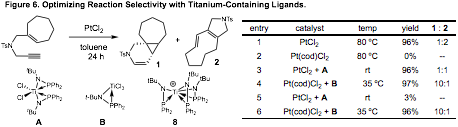
On advantage of achieving high reactivity with our bimetallic complexes in platinum catalysis is the opportunity to use ligand structure to control product selectivity. Cycloisomerization reactions with platinum catalysts often react to give a mixture of diene and cyclopropane products. In the absence of useful ligands for this process, optimizing reaction selectivity is not possible. In applying our titanium-containing ligands to this process, we achieve high reactivity via metal-metal interactions, and then use the ligands structure to influence selectivity. In these studies, we demonstrated that our platinum chloride gave poor product selectivity in the cycloisomerization reaction (Figure 6). When our titanium-containing ligands were employed, however, high selectivity for the cyclopropane product could be observed.
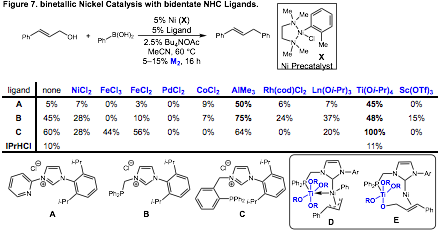
Heterobimetallic Nickel Catalysis: The results presented above focus on the use of titanium-containing ligands for heterobimetallic catalysis. We have recently initiated efforts to develop bidentate N-heterocyclic carbene (NHC) ligands for bimetallic catalysis. Our hypothesis is that a NHC-metal complex containing a labile coordinating group could recruit a second metal and enable bimetallic catalysis. This would allow us to screen second metal effects in catalysis. In our initial efforts, we have synthesized various bidentate NHC ligands and screened their activity in nickel-catalyzed Suzuki reactions with simple allylic alcohols. Importantly, this transformation has not been reported with nickel catalysts, giving us an opportunity to explore new reactivity with nickel catalysts.
In our optimization studies, we screened a variety of metal precursors in combination with our nickel NHC complexes. Importantly, we found several positive effects when Lewis acidic metals such as aluminum and titanium were employed. We were intrigued by that fact that many of the second metal effects, such as with titanium, iron, and aluminum were actually ligand dependent. This suggests that the ligand structure is important to influencing the impact of the second metal in catalysis. In particular, we observed that with ligand C, we could obtain 100% conversion to the cross coupled product when titanium isopropoxide was employed. While we do believe that activation of the alcohol substrate by titanium is likely increasing the rate of catalysis, we are currently investigating the formation of bimetallic complexes such as D and E to determine if the ligand scaffold is increasing reactivity via interactions with nickel (D) or by binding to and activating the alcohol substrate in close proximity to nickel (E).
Conclusion: The results presented above confirm the potential of heterobimetallic catalysts to contribute new and important reactivity to organic transformations. We have also demonstrated several strategies for employing heterobimetallic catalysis in organic reactions, and have developed new methods for enantioselective catalysis with heterobimetallic complexes.