Reports: DNI1055526-DNI10: Effects of the Chemistry and Structure of Petrochemical Polymers on the Vapor-Phase Conversion to Organic-Inorganic Hybrid Materials
Mark D. Losego, Ph.D., Georgia Institute of Technology
This ACS PRF DNI award supports fundamental research into vapor phase infiltration (VPI), a new chemical vapor processing technique used to transform petrochemical polymers into organic-inorganic hybrid materials with unique properties. This research program is specifically focused on clarifying the processing kinetics of VPI and developing techniques to quantitatively measure those kinetics.
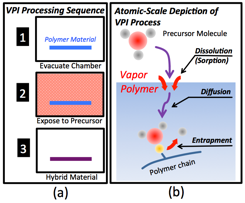
During Year 2 of this Doctoral New Investigator Award, the PI and his supported students have worked to create a mathematical model to describe the VPI kinetics. As pictured in Fig. 1, this model has three parts: (1) dissolution of the precursor into the polymer, (2) diffusion of that precursor throughout the polymer, and (3) entrapment (via, e.g., reaction) within the polymer.
Fig. 1: (a) Schematic of VPI process. (b) Depiction of the atomic scale processes important in VPI.
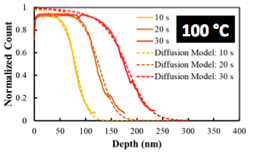
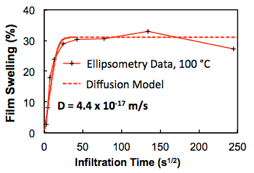
Using Fick's 2nd Law to describe the diffusional transport processes, two equivalent solutions can be written: (Case 1) the concentration profile as a function of depth at fixed times and (Case 2) the total mass uptake of the polymer as a function of time. These two solutions can be used to extract an effective diffusion coefficient from different types of experimental data. For example, Case 1 can be used to extract diffusivities from secondary ion mass spectrometry (SIMS) composition profiles, as pictured in Fig. 2, while Case 2 can be used to extract diffusivities from film swelling data collected via spectroscopic ellipsometry as pictured in Fig. 3. In the latter case, we use the swollen film's thickness as a proxy for mass uptake. Both of these examples use trimethylaluminum (TMA) VPI of PMMA as the model test system.
Fig. 2: Plot of Al concentration as a function of depth in a PMMA film VPI with TMA at 100°C as measured by SIMS. Several exposure times (10 s, 20 s, 30 s) are shown. This data is fit to a diffusion model describing concentration as a function of depth.
Fig. 3: Plot of PMMA film swelling as a function of TMA VPI process time at 100 °C as measured by spectroscopic ellipsometry. This data is fit to a diffusion model describing total mass uptake as a function of time.
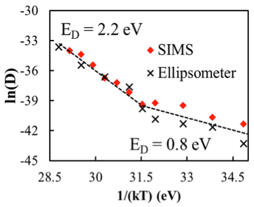
By collecting this diffusional data at different temperatures, we are able to create an Arrhenius plot, as shown in Fig. 4. Evident in this plot is the consistency in diffusivity values measured with SIMS and ellipsometry. In this plot we note a change in the slope. We currently attribute this change in slope to represent a transition from a purely diffusion controlled process to a process that has mixed diffusion and reaction character. At lower temperatures, the activation energy represents the energy barrier to diffusion (0.8 eV) while at higher temperatures, the effective diffusion coefficient is reduced due to reactions. The change in slope can be equated to the free energy for this reaction, approximately -135 kJ/mol.
Fig. 4: Arrhenius plot of measured TMA effective diffusivities in PMMA as a function of temperature. Plot shows diffusivities measured using both SIMS depth profiles and ellipsometric analysis of film swelling.
We are now building a VPI chamber with a quartz crystal microbalance to do in situ gravimetry measurements that track total mass uptake as a function of time. This data collection will be more rapid than current ex situ experiments, accelerating our exploration of how different polymer and precursor chemistries affect the VPI process kinetics.
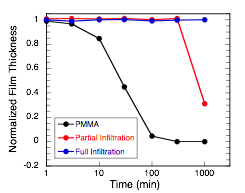
We also continue to explore the chemical resistant properties of VPI modified polymers. Fig. 5 plots the normalized thickness of treated and untreated 1.4 micron thick PMMA films spun cast on silicon exposed to toluene for extended periods of time. Evident is the rapid dissolution of the untreated PMMA material (within 2 hours) versus the nearly full retention of VPI treated PMMA films for a period of >1 day. These new hybrid materials are found to have similar robustness even when expose to heated (60 °C) toluene. Using our understanding of VPI processing kinetics, we can also partially infiltrate the polymer and create a material with intermediate chemical resistance.
Fig. 5: Plot of normalized thickness versus log-scale time for 1.4 mm films on silicon immersed in toluene. Three films are shown: PMMA, PMMA partially infiltrated using TMA VPI and PMMA fully infiltrated with TMA VPI.
Impact of Funding on PI's Research Program:
Generous support from this ACS PRF Doctoral New Investigator Award has benefited both the PI's career trajectory and his students' educational progress. The graduate student supported on this project has successfully passed his Ph.D. proposal, presented a talk about his research at the 2016 AVS conference, and received a graduate Fellowship from Georgia Tech's Center for the Science and Technology of Advanced Materials and Interfaces. Preliminary results from this project also contributed to the PI's selection for the 2017 3M Non-Tenured Faculty Award.