Reports: DNI655481-DNI6: Elucidating Support Effects and Size Effects of the MoS2-Based Catalysts from First-Principles Calculations
Kesong Yang, PhD, University of California, San Diego
Narrative Progress Report
1. Summary of Grant Activities
In the past grant period, 01-Sep-16 to 31-Aug-17, the major research grant activity is devoted to elucidating the size effects and odd-even effects in the MoS2 nanosheets, as well as their implications in the catalytic applications from first-principles total energy calculations. As detailed below, based on the first-year’s achievements, this grant continues producing high-quality research, academic, and education achievement. One research article is currently under the review, and several other manuscripts are under the preparations.
2. Accomplishment of Research Activities
The research accomplishments are detailed as below:
a) Research Results, Conclusions, and Next-Step Research Plan
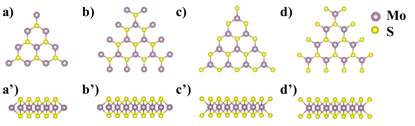
In the first year of the grant period, we focused on the computational/theoretical understanding of the size effects of 2D MoS2 nanosheet and the odd-even effects in the formation of sulfur vacancies in these nanosheets. For instance, Figure 1 shows the single-layer MoS2 in a triangle shape. In this work, we have modeled four types MoS2 edge structures with zig-zag pattern, defined as ZZ-Mo-1, ZZ-Mo-2, ZZ-S-1, and ZZ-S-2, respectively.
Figure 1. Single-layer MoS2 nanosheets (n=4) with zig-zag patterned edges from top view (a-d) and side view (a’-d’), respectively. (a,a’) ZZ-Mo-1, (b,b’) ZZ-Mo-2, (c,c’) ZZ-S-1, and (d,d’) ZZ-S-2. The large purple and small yellow balls represent Mo and S atoms, respectively. n standards for the number of Mo atoms in the edge of the MoS2 nanosheet.
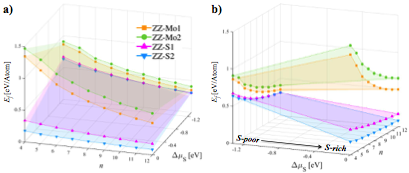
The calculated formation energies of the four types of MoS2 nanosheets, ZZ-Mo-1, ZZ-Mo-2, ZZ-S-1 and ZZ-S-2, as a function with the reference chemical potential of S, ∆μS, and the nanosheet size, n, are plotted in Figure 2. Several important trends are revealed as below:
(i) As the size of MoS2 nanosheet (n) increases, the formation energy of all the four types of MoS2 nanosheets decreases, indicating that larger MoS2 nanosheets are energetically more favorable to form, see Figure 2a.
(ii) The S-terminated nanosheets (ZZ-S1 and ZZ-S2) have lower formation energies than Mo-terminated ones (ZZ-Mo1 and ZZ-Mo2) in the whole range of the ∆μS, and they have maximum formation energy difference un- der the S-rich condition.
(iii) The ZZ-S2 models are energetically more favor- able to form than ZZ-S1 structures for most range of ∆μS, though they tend to have similar formation energies under extremely S-poor condition.
Figure 2. Calculated formation energies of ZZ-Mo1, ZZ-Mo2, and ZZ-S1, and ZZ-S2 nanosheets with respect to the size (n) and chemical potential of sulfur (∆μS), plotted from viewing angle of a) size, n and b) ∆μS.
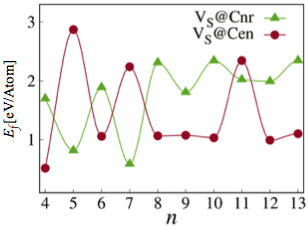
In addition, we also revealed an odd-even effect of the formation of sulfur vacancies in the MoS2 nanosheets. The calculated formation energies of ZZ-S2 MoS2 nanosheets with sulfur dimer vacancy as a function of the size (n) of the nanosheets is shown in Figure 3. The following trends can be found:
i) At 4 ≤ n ≤ 8, i.e., for small MoS2 nanosheets, the VS@Cen dimer vacancy has a low formation energy for even-number size but high formation energy for odd- number size, while the VS@Cnr dimer vacancy has a complete opposite trend. These results indicate a strong odd-even effect in the formation of sulfur vacancies in the small MoS2 nanosheets and the energetic preference for the location of sulfur dimer vacancies.
ii) At 9 ≤ n ≤ 13, i.e., for relatively large MoS2 nanosheets, the two types of dimer vacancies basically follow the same trend with that at 4 ≤ n ≤ 8, but the odd-even effect becomes relatively weak.
iii) For VS@Cnr dimer vacancy, as n increases, its formation energy at 9 ≤ n ≤ 13 is generally larger than that at 4 ≤ n ≤ 8. This indicates that the VS@Cnr dimer vacancies tend to be formed in the small MoS2 nanosheets.
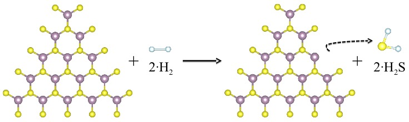
On the basis of the odd-even effects in the formation of sulfur vacancies, we also found a similar odd-even effects in the hydrodesulfurization (HDS) reaction. The scheme of the HDS reaction on the ZZ-S2 type MoS2 nanosheet is shown in Figure 4.
Figure 3. Calculated formation energies of ZZ-S2 MoS2 nanosheets with sulfur dimer vacancy as a function of the size (n) of the nanosheets. Blue line with triangular points represent VS@Cnr and red line with circular points represent VS @Cen.
Figure 4. Scheme of the HDS reaction on the ZZ-S2 type MoS2 nanosheet. One sulfur dimer reacts with two H2, forming one dimer sulfur vacancy and two H2S molecules.
b) Research and Education Achievements
The research project under the grant has a significant positive impact on the PI’s academic career. The grant helps further establish the PI’s research group, and expand the PI’s research direction in the materials applications of petroleum industry. One important goal of this project is to develop a scientific workforce to the petroleum-related research. Hence, the grant also significantly influences the students (graduate student Mr. Paul Joo, Mr. Jianli Cheng, and undergraduate students Miss Camille Bernal, Mr. Joseph Wong) by providing them research opportunities and introducing them the frontiers of petroleum research.
i) Original Peer-Reviewed Work
Paul H. Joo, Maziar Behtash, and Kesong Yang*, Energetic Stability, Oxidation States, and Electronic Structure of Bi-doped NaTaO3: First-Principles Hybrid Functional Study, Phys. Chem. Chem. Phys. 18, 857-865, (2016).
Paul H. Joo, Jianli Cheng, and Kesong Yang*, Size Effects and Odd-Even Effects in the MoS2 Nanosheets: First- Principles Studies, (Under Review)
ii) Students’ Research Award and Achievements
Joseph Wong, First-place poster award in the 2017 Materials Research Society Spring Meeting
Paul Joo, GSA Travel Grant for 2016 MRS Spring Meetings & Exhibits
Paul Joo, Best Poster Award, 2016 First Southern California Theoretical Chemistry Symposium
Camille Bernal was admitted to graduate school of California Institute of Technology (Caltech)
Miss Cruz was admitted to the graduate school at UC Riverside
iii) Abstract Presented in Conference
2016 MRS Spring Meetings & Exhibits, Phoenix, AZ. Paul Joo, Mazir Behtash, and Kesong Yang, Energetic Stability, Oxidation States, and Electronic Structure of Bi-doped NaTaO3: First-Principles Hybrid Functional Study.