Reports: DNI1054898-DNI10: Corrosion in Extreme Environments
Hojong Kim, PhD, Pennsylvania State University
Introduction.
Exploration and extraction of oil and gas wells require the use of corrosion-resistant materials that can withstand harsh chemical surroundings containing hydrogen sulfide (H2S). In H2S-containing sour environments, steels are susceptible to abrupt mechanical failures, known as sulfide stress cracking where hydrogen atoms as a product of the corrosion process diffuse into the base metal and embrittle the crystalline structure. In sulfide stress cracking, sulfide films (e.g., FeS2) are also present at the metal/environment interface as corrosion products. However, the mechanistic role of these film in interfacial corrosion processes (e.g., hydrogen reduction reactions) is not fully understood, possibly due to their complex phase structures. Enabled by this ACS PRF grant, this study aims to understand the interfacial and transport properties of hydrogen through the steel membrane in H2S-containing aqueous environments.
Methods and Results.
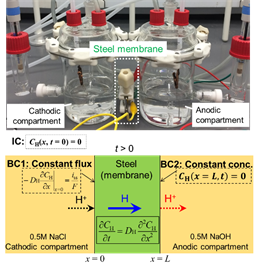
In the last year, the transport properties of hydrogen were studied using a Devanathan-Stachurski electrochemical permeation cell [1], in which the current (hydrogen flux) and the potential (hydrogen surface concentration) are controlled at both bipolar membrane interfaces simultaneously using high-accuracy bi-potentiostats (Figure 1). The permeation cell was comprised of (1) a cathodic compartment where hydrogen atoms are electrochemically charged (reduced) at the membrane surface in an aqueous solution (deaerated, pH 3–13) and (2) an anodic compartment where hydrogen atoms exit the membrane surface by oxidizing electrochemically (0.1M NaOH). The exit side of the steel membrane (hydrogen oxidation) was sputter-coated with Pd (20–30 nm) to facilitate the selective oxidation of hydrogen while preventing the dissolution of iron.
Figure 1. Two-compartment hydrogen permeation cell for the study of H-transport properties (top), and schematic illustration of transport kinetics with boundary (BC) and initial (IC) conditions at the steel membrane surfaces during the electrochemical measurements (bottom).
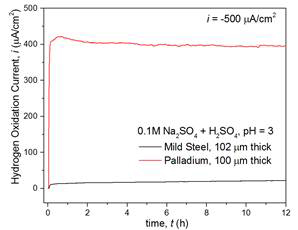
The hydrogen transport properties were investigated under the control of solution pH in the cathodic compartment, using NaOH (0.1 M, pH = 13), Na2SO4 (0.1 M, pH = ~7), and Na2SO4 + H2SO4 (0.1 M, pH = 3) at various membrane thicknesses (25–178 µm). The hydrogen permeation current density exhibited essentially equivalent transient behavior for 32 h in three different pH solutions in cathodic compartment (Figure 2a), indicating the diffusion-controlled hydrogen transport through the membrane (102 µm). The observed permeation current density through the steel membrane was at ~22 µA/cm2, far less than Pd membrane at ~400 µA/cm2 (Figure 2b) due to the limited solubility of hydrogen in mild steels.
Figure 2. Hydrogen permeation current density, (a) through mild steel membrane (102 µm) at selected solution (pH 3–13), and (b) comparison with pure Pd membrane (100 µm) at pH 3 solution. For both (a)-(b), constant hydrogen charging current density was maintained at i = ‒500 μA/cm2 at the cathodic interface (galvanostatic charging) while hydrogen oxidation was potentiostatically controlled at E = +0.3 V vs. Ag/AgCl at the exit side.
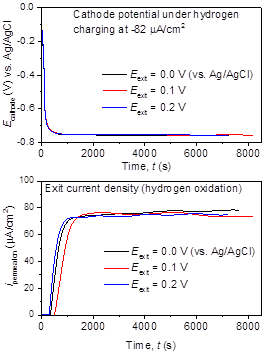
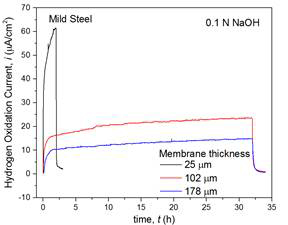
Using Pd as a base, the diffusivity of hydrogen was estimated to 1.30–1.70 × 10-7 cm2/s, based upon the transient permeation current density (Figure 3a), in excellent agreement with the reported values at 1.30–3.40 × 10-7 cm2/s at room temperature [1-3]. Similarly, the transient permeation currents through the steel membranes at 25–178 µm thickness were analyzed (Figure 3b) and the effective diffusivity was estimated at ~1.1±0.5 × 10‒7cm2/s, which is under further verification and comparison with the reported values in the literature. Based on these results, the research is in progress to understand the effect of dissolved H2S in hydrogen reduction and recombination kinetics as well as the resultant permeation properties. The H2S will be introduced into the solution phase by dissolving the controlled quantities of sodium sulfide (Na2S) in the acidic solution of H2SO4 (Na2S + H2SO4 → H2S + 2Na2SO4).
Figure 3. (a) the electrode potential of Pd membrane (100 µm) with time under constant current density at 82 µA/cm2 (top), and the transient hydrogen oxidation current density in the anodic compartment (bottom), and (b) hydrogen permeation current through mild steel membranes (25–178 µm) under constant current charging current density, i = ‒500μA/cm2.
Impacts of Research.
This study will establish the chemical interactions between the steel and sour environments as well as the hydrogen transport properties in the presence of H2S molecules, which will serve as a basis in understanding the degradation reactions of engineering materials for better design of corrosion-resistant coatings. The ACS-PRF grant enabled the PI to initiate fundamental corrosion research in extreme environments, to build essential laboratory infrastructure, and to educate and train both the undergraduate and graduate students. Electrochemical investigation of materials coupled with the chemical/structural characterization will enrich the students’ knowledge for their professional career development as scientists and engineers. Under this work, two undergraduate students completed their senior theses and a graduate student is pursuing doctoral research with the financial support from this grant.
References.
[1] M. A. V Devanathan and Z. Stachurski, “Adsorption and Diffusion of Electrolytic Hydrogen in Palladium,” Proc. R. Soc. London Ser. a-Mathematical Phys. Sci., vol. 270, no. 1340, pp. 90–102, 1962.
[2] J. G. Early, “Hydrogen diffusion in palladium by galvanostatic charging,” Acta Metall., vol. 26, no. 8, pp. 1215–1223, 1978.
[3] R. V. Bucur, “Measurements of diffusion coefficients of hydrogen in palladium by a galvanostatic permeation method,” Int. J. Hydrogen Energy, vol. 10, no. 6, pp. 399–405, 1985.