Reports: UR356706-UR3: Neutral Dinuclear Iron Complexes Supported by N-amido-Metal Bonds
Radu F. Semeniuc, Eastern Illinois University
Overview
The goal of this project was to prepare a series of metal complexes (with a focus on the Fe(II) metallic center), then investigate their propensity activate molecular oxygen and, in the case of iron, to form oxygen adducts. The ligands we proposed to use for this chemistry are built around a Namido-Ophenoxo-Namido core, having either the N-H or C=O group directly attached to the phenyl ring, respectively. Their side-arms are further functionalized with a bis(pyrazolyl)methane donor set.
The specific objectives of this project were:
1. Synthesis and characterization of the ligands.
2. Synthesis and characterization of Fe(II) dinuclear complexes.
3. Synthesis and characterization of high-valent diiron-oxo complexes.
Progress report
We have successfully completed the first specific objective, by preparing the ligands H3L1 and H3L2 depicted in Figure 1. H3L1 was prepared from bis(pyrazolyl)acetic acid and 4-t-butyl-2,6-diaminoanisole, followed by demethylation of the anisole with BBr3. H3L2 was prepared from the reaction of 2-hydroxy-4,6-dimethylisophthalic acid with 1,1’-bis(pyrazolyl)ethanamine, in the presence of P(OPh)3 in pyridine as solvent. Both ligands are white powders and they were characterized by 1H- and 13C-NMR and IR spectroscopy.
Figure 1. Descriptive structures of the H3L1 and H3L2 ligands.
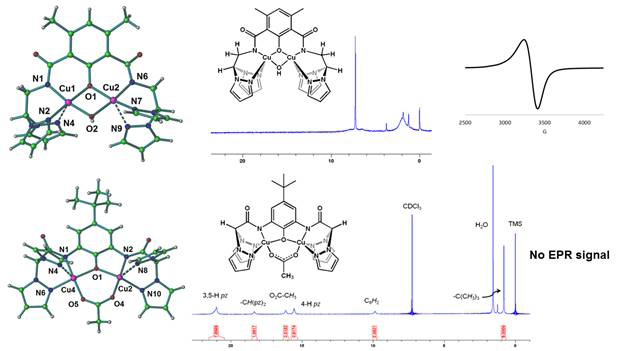
We have also made progress with the second specific objective of this project. We have prepared the copper(II) complexes of these ligands, and characterized them by IR, UV-Vis, 1H-NMR, and EPR spectroscopies, cyclic voltammetry and single crystal X-ray diffraction (see Figure 2). In both complexes, the copper ions were found in square pyramidal geometries. Due to the different position of the amido NH region within the ligand structure, in the L2Cu2(OH) complex, the Cu(II) ions are part of six membered metallacycles, while in L1Cu2(OAc) five membered metallacycles are formed. As a consequence, different Cu ··· Cu distances (2.966 Å and 3.519 Å, respectively) were identified in their solid-state structures.
Figure 2. Structural (X-ray diffraction) and spectroscopic (NMR and EPR) characterization of L2Cu2(OH) (top) and L1Cu2(OAc) (bottom).
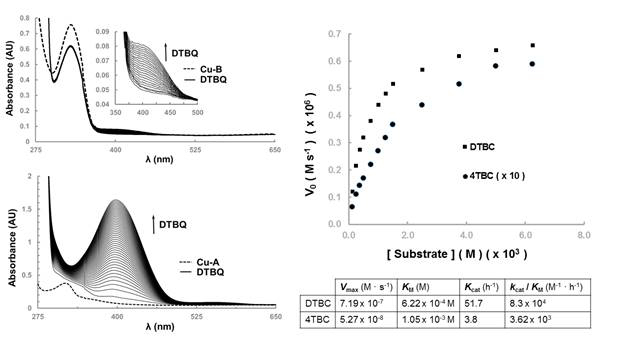
We have studied the catechol oxidase activity of these complexes in dioxygen-saturated methanol, and found that while L1Cu2(OAc) complex displays little activity (Figure 3 left – top), L2Cu2(OH) shows appreciable catalytic properties (Figure 3 left – bottom). The latter complex oxidizes 3,5-di-tert-butylcatechol (DTBC) and 4-tert-butylcatechol (4TBC) to their corresponding quinones, but fails to oxidize 4-nitro-catechol and pyrocatechol. The catalytic activity of L2Cu2(OH) follows a Michaelis-Menten type kinetics, as shown in Figure 3 right.
Figure 3. Left: catechol oxidase activity (monitored by UV-Vis spectroscopy) of L1Cu2(OAc) (top) and L2Cu2(OH) (bottom); spectra recorded every 2 minutes; right: kinetic parameters for the oxidation of DTBC and 4TBC by L2Cu2(OH).
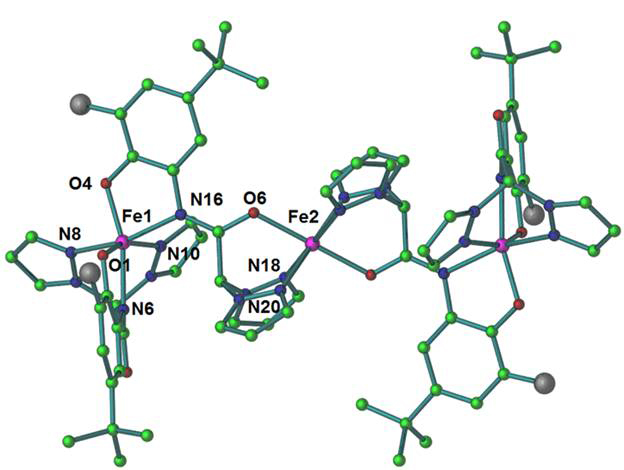
We have also tested the reaction between these ligands and Fe(II) centers. Toward this end, we report the synthesis of an unusual trinuclear mixed valence Fe(II)-Fe(III) complex, L14Fe3, pictured in Figure 4, obtained by the reaction between H3L1, Fe(BF4)2·6(H2O) and KOH under aerobic conditions. The complex is formed by the coordination of four L1 ligands to the iron centers. Each ligand is involved in the coordination with only one side-arm; the other arm (not coordinated) is depicted as a grey sphere. The complex resides on an inversion center situated on the Fe2 center. One ligand coordinates to the Fe(III) ion (Fe1 in the Figure) via its deprotonated amide nitrogen atom and the phenoxo group, and the bis(pyrazolyl)methane donor set. The remaining coordination sites of Fe1 are filled by the deprotonated amide nitrogen atom and the phenoxo group of the second L1 ligand. The amide oxygen atom and the bis(pyrazolyl)methane groups of this second ligand coordinates to the Fe(II) bridging center, thus forming the trinuclear complex. This structure contrasts with those obtained for copper, and highlights the different chemistry of iron. To obtain the desired dinuclear iron(II) complexes and study their interaction with molecular oxygen, we will perform these reactions under inert atmosphere and use non-protic solvents.
Figure 4. The molecular structure of the L14Fe3 complex; the grey sphere represents the uncoordinated side-arms of the ligands (removed for clarity purposes).
Impact of funding
Funding of this proposal had an important impact on the PI’s career. The research component of this project provided the PI with the opportunity to study and understand the factors that govern the synthesis and characterization of metal complexes supported by Namido-Metal bonds. It also provided him with the opportunity to engage in new collaborations with research groups having similar research interests from other Universities.
During the summer of 2017, four students (two undergraduate and two masters) were supported by this grant. During their time in the PI’s lab, the students were trained in synthetic design, and accumulated experience in ligand and metal complex syntheses (including those requiring the handling of air and moisture sensitive compounds), instrument operation, and spectral interpretation. In the same time, they were able to identify, acquire and describe information pertaining to the synthetic and characterization methods used in this particular field, and integrate the principles learned in various chemistry lectures with the laboratory experiences. The experiments performed by the PI’s students reinforced a broad range of fundamental principles covered in regular inorganic, organic, and physical chemistry courses.
The results of this project have been disseminated through presentations at regional meetings. The PI was invited to give a talk at the 68th Southeastern Regional Meeting of the American Chemical Society (October 23-26, 2016). One student presented a poster at Eastern Illinois University’s Student Research and Creative Discovery Conference (Friday, Mar 31, 2017). Furthermore, two papers are in preparation and will be submitted soon.