Reports: UR355441-UR3: Synthesis, Structural, and Spectroscopic Studies of Porphyrin Based Model Asphaltene Lanthanide and Group 11 Bimetallic Complexes: Intra- and Inter-Molecular Excimers and Exciplexes Studies in Solution and Solid State
Zerihun Assefa, North Carolina A&T State University
Introduction
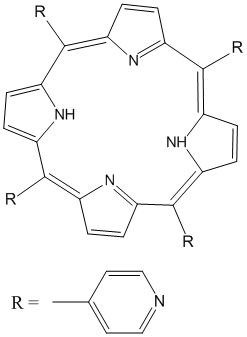
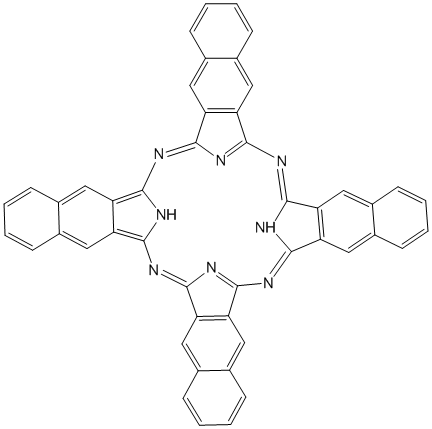
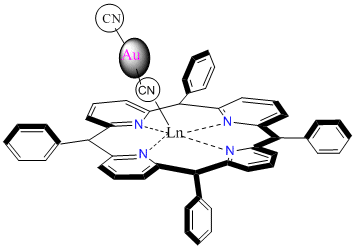
We have continued exploring porphyrin based model complexes that will be used to simulate asphaltene aggregations. The ultimate research goal of this project is the synthesis and characterization of materials involving bimetallic systems containing group 11 transition metals (in particular cyanoaurate complexes) and selected lanthanide ions. Asphaltenes are known as the portion of crude oil insoluble in light n-alkanes (such as n-heptane), but soluble in aromatic solvents [1]. They consist of a complex mixture of heavy organic molecules containing fused polyaromatic systems, pendant alkyl chains, polar functional groups with O, N, and S atoms and metals, such as V, Ni, and Fe [2]. Hence, asphaltenes can be considered to comprise the most polar fraction of crude oil. Although asphaltenes are considered problematic in crude oil compositions, the industry has been showing interest in their utility because of their high composition (~15%) in crude oil. Hence, a deeper understanding of asphaltenes is required to make this shift in the industry. While asphaltenes structures are some how complicated and believed to incorporate four-to-ten fused aromatic rings, with porphyrins at the core. Porphyrins are versatile molecules present in natural systems and are well known to act as ligands binding metals and form complexes [4]. This study incorporates porphyrin core attached with extended aromatic rings in order to simulate that of asphaltenes’ structure. Lanthanide metal ions will be incorporated in the porphyrin core (Figure 1) followed by linking the core with group 11 cyanide ions as shown in Figure 2.
Figure 1: Examples of peripheral substituted ligands that can be used as models for assessing pi-pi interactions.
Figure 2: Example of expected bimetallic complex with peripheral substituted ring for potential pi-pi interaction and possible excimer and/or exciplex formation.
The Gd(III) ion in particular is targeted in this study since lanthanide porphyrin complexes with open shell electronic configuration usually show weak florescence assignable to the phorphyrin ring. The characteristic f-f emissions expected from the lanthanide ions are heavily quenched even in the case of some of the strong emitters such as Tb3+ and Eu3+ ions. This phenomenon is largely attributed to excited state energy transfer to the porphyrin ring that quenches the lanthanide. Due to the large f-f gap the Gd(III) emission is expected unaffected by excited energy transfer to the porphyrin ring, hence would function as a reference for the other lanthanide incorporation including Yb, Nd, and Er.
Methodology
All chemicals were purchased Sigma Aldrich and used without further purification. The synthesis of Gadolinium tetraphenylporphyrin complex was conducted by mixing 100 mg of Gd(NO3)3* 6H2O with 135.9 mg of tetraphenylporphyrin in 10 mL of chloroform. The deep dark solution was refluxed for 2 hours while stirring. No color change was observed throughout the refluxing step. The dark solid product was collected after removing the solvent with rotary evaporator. Attempt for crystal growth suitable for x-ray diffractometer was unsuccessful thius far.
Similarly synthesis of gadolinium protoporphyrin complex was conducted as described above where 100 mg of Gd(NO3)23* 6H2O and 124.8 mg protoporphyrin were used. The bimetallic silver- gadolinium protoporphyrin complex was synthesized by mixing 20 mg of the gadolinium protoporphyrin powder with 4 mg of potassium dicyanoargentate in 5mL of acetonitrile. The flask was covered with a septum and stirred for 3 hours. The solution was then filtered, washed and the solid product with dried in open air.
The dicyanoargentate ion is used during the synthesis because it is known to form a CN bridged coordination polymeric system and/or bi-metallic systems with lanthanide ions. In this study it specifically serves as a spacer, restricting involvement of the porphyrin core from π-π overlap interactions. Hence it could be possible to isolate the possible π-π interaction on the peripheral aromatic substituents that will be used as a model for asphaltenes. The reaction progress was monitored using IR studies that allowed us to follow the bonding comparison as the reaction progresses.