Reports: DNI756380-DNI7: Controlling Polarization in Polystyrene
Rebekka Klausen, Johns Hopkins University
1. Research Impact
Polystyrene (PS) is an essential commodity petrochemical produced annually on massive scale. Work in the Klausen group in the last year has focused on identifying ways of controlling the incorporation of polar functionality into polystyrene, with the goal of developing materials with tunable polarity and wettability. PS derivatives arising from chemical modification of the benzene ring are important materials, such as polystyrene sulfonate, the ion exchange resin Dowex.
The incorporation of boron into organic materials for functional purposes is an active area of synthetic innovation. BN for CC bond substitution is an attractive approach to developing stable organoboranes. The BN bond is isosteric and isoelectronic with the CC bond, but unlike the CC bond, the BN bond is polar. Elucidation of the consequences of BN bond polarization on bulk properties requires scalable synthetic approaches. A full exploration of synthesis-structure-property relationships also demands multigram scale synthetic approaches.
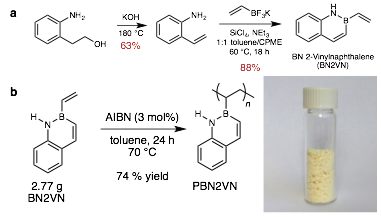
Figure 1. a) Two step, high yielding synthesis of BN2VN. b) Multigram scale BN2VN homopolymerization. Inset shows vial of PBN2VN.
Earlier this year we reported the two-step synthesis of BN2VN, a novel boron-containing vinyl monomer (Figure 1a) (Chem. Commun. 2017, 53, 7262). The short, high yielding synthesis enables the multigram radical polymerization of BN2VN (Figure 1b). This is the first access to multigram quantities of a boron-containing polymer, whereas others have described milligram scale polymerizations of related structures.
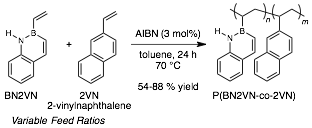
We studied the copolymerization of BN2VN and the hydrocarbon analog 2-vinylnaphthalene (2VN) (Figure 2). These studies were undertaken to understand the relative rates of BN2VN and 2VN reactivity. Our data are consistent with similar reaction rates under free radical conditions and strongly suggest that BN2VN should readily form copolymers with other petroleum-derived olefin monomers. As support for the claim that BN2VN and 2VN have similar reaction rates, we show a close agreement between the monomer feed ratio and the incorporation of BN2VN (in weight percent, wt. %). To do this, the Klausen group developed innovative methods for the quantitative characterization of BN2VN incorporation into polymeric structures, including a simple absorbance spectroscopy assay based on the unique photophysical properties of BN2VN. Table 1 summarizes the molecular weight characteristics, optical properties, and BN2VN content of the homopolymers and copolymers.
Figure 2. Free radical co-polymerization of BN2VN and 2VN. AIBN = 2,2-azobis(2-methylpropionitrile).
The controlled incorporation of the polar aromatic monomer BN2VN into polystyrene results in tunable physical properties. In our investigation of BN2VN optical properties, we have demonstrated a linear relationship between extinction coefficient at 320 nm and BN2VN content. Polystyrene derivatives with controlled content of an orthogonal chromophore may find utility as photoresists (UV curing). Additionally, due to its lack of symmetry and the polar BN bond, BN2VN is more polar than hydrocarbons like styrene and 2VN. The incorporation of this monomer is expected to result in a controlled change in water contact angle and solubility in polar, protic solvents. Investigation of these physical properties of our BN2VN copolymers is ongoing.
Table 1. Optical and molecular weight characteristics of polymers and copolymers.
Entry | Sample Namea | Feed Ratio BN2VN:2VN | ε320 (L g-1 cm-1)b | BN2VN (wt. %) | Mn (kDa)c | Mw (kDa)c | ĐM |
1 | PBN2VN | 100:0 | 30.7 | 100 | 6.04 | 8.66 | 1.43 |
2 | P(BN2VN74-co-2VN26) | 80:20 | 23.4 | 74 | 6.49 | 8.73 | 1.34 |
3 | P(BN2VN49-co-2VN51) | 50:50 | 16.6 | 48 | 6.22 | 9.22 | 1.48 |
4 | P(BN2VN9-co-2VN91) | 20:80 | 5.38 | 9.2 | 7.12 | 11.0 | 1.54 |
5 | P2VN | 0:100 | 2.86 | 0 | 8.36 | 14.0 | 1.68 |
a Samples named according to experimentally determined wt. % BN2VN and 2VN incorporation. b See Chem. Commun., 2017, 53, 7262-7265 for details. c Measured by gel permeation chromatography (GPC) at 254 nm relative to polystyrene standard (THF, 0.35 mL min-1, 40 °C). | |||||||
The exciting results of BN2VN and 2VN copolymerization under free radical conditions suggests further opportunities for characterizing synthesis-structure-property relationships. Recently, we have prepared copolymers of BN2VN and styrene itself. As before, an excellent agreement between wt. % BN2VN in the isolated copolymer and the feed ratio suggests very similar reaction rates. We have also begun an investigation of the use of PBN2VN as an intermediate in the preparation of copolymers that are challenging to access by typical synthetic methods. Two publications on these results are planned for the next year, one describing copolymerization with styrene and another postpolymerization modification of PBN2VN. Work is also planned that focuses on controlled radical polymerization methods for block copolymer synthesis and single site catalytic approaches to BN2VN polymerization.
2. PI Career Development
In the 2017 funding period, Prof. Klausen presented invited seminars at five conferences and three universities. Her PRF-supported research was featured in all eight seminars. Two of the seminar invitations included recognition of Prof. Klausen as a leading early career researcher: she was named a featured Young Investigator by both the Polymeric Materials: Science and Engineering (PMSE) and the Organic (ORGN) divisions of the American Chemical Society (Spring 2017 and Fall 2017 ACS meetings, respectively).
The PI was recognized by two other awards in the 2017 funding period. The Alfred P. Sloan Foundation named her a 2017 Sloan Research Fellow. She also won the 2016 Braude Award for outstanding research with undergraduate students, sponsored by the Maryland section of the American Chemical Society. This award recognized that undergraduates in the Klausen group have unique opportunities to perform outstanding research. Mr. Jae Young Lee, a current senior, received the prestigious Provosts Undergraduate Research Award (PURA) from Johns Hopkins University for his work on PBN2VN. He is also a co-first author with Ms. Heidi van de Wouw on our published work. Prof. Klausen has submitted a proposal to the NSF to continue research in this area.
This PRF-supported research on PBN2VN and derivatives was featured in the Emerging Investigators-themed issue of the journal Chemical Communications.