Reports: UNI156004-UNI1: Benzodiazaboroles as Potential Dynamic Covalent Functional Groups for New Molecular Architectures
Dustin E. Gross, PhD, Sam Houston State University
In the first year of this grant, we have focused on the synthesis of novel boronic acid–based derivatives. Specifically, we have exploited the pinacol rearrangement in the interchange of boronate esters, synthesized new benzoxazaboroles and benzodiazaboroles from 2-(alkylamino)phenols and alkyl benzene diamines, respectively, and made progress toward synthesizing diazaborole based macrocycles.
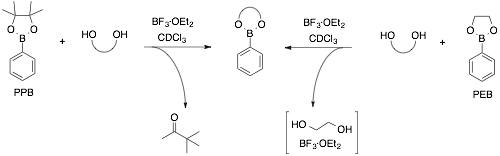
Boronate ester interchange: In this project, we have focused on the transesterification of boronate esters with selected diols. A key finding is that BF3·OEt2 facilitates the conversion of pinacol (PPB) and ethylene glycol (PEB) boronate esters to other boronate esters. While we have evidence that the conversion of the PPB ester involves the consumption of pinacol via the pinacol rearrangement, we believe the PEB conversion involves the complexation of ethylene glycol and BF3. We plan to use this methodology in the construction of oligomeric materials, as pinacol esters are typically more synthetically accessible intermediates.
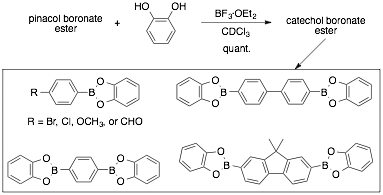
We have also extended this methodology to several bis-boronate esters and studied the BF3·OEt2 facilitated pinacol rearrangement on a series of alkyl 1,2-diols including 1-phenylethane-1,2-diol and meso-hydrobenzoin.
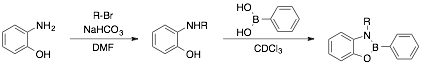
Oxazaboroles: Oxazaboroles are structurally similar to dioxaboroles in that one of the oxygen atoms is replaced with nitrogen. Because of their structural similarities, oxazaboroles may also have the potential to be used in dynamic covalent oligomeric systems. In this work, we have synthesized and characterized a series of N-alkylbenzoxazaboroles from the corresponding N-alkylaminophenols.
We have found that oxazaboroles readily self-assemble in various solvents. Specifically, dissolving a phenyl boronic acid along with a 2-(alkylamino)phenol in ethyl acetate and removing the solvent at room temperature results in the quantitative formation of the corresponding benzoxazaborole.
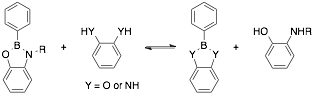
Benzodiazaboroles: In this project, we have continued our efforts to understand diazaborole formation and stability by studying the formation in several solvents. We have found that by dissolving phenyl boronic acid and benzene-1,2-diamine in various high-boiling (>100 °C at 1 atm) solvents and then removing the solvent at the minimum required temperature on the rotary evaporator results in the quantitative formation of diazaborole. We have also characterized diazaboroles by X-ray crystallography and performed density functional calculations on several diazaborole analogues.
Furthermore, we have synthesized several alkyl and dialkyl benzene diamines and subsequently reacted them with boronic acid derivatives to form N-alkyldiazaborole derivatives. We have run some preliminary equilibration studies with 2-phenyl-1,3,2-benzodiazaborole and alkyl benzene-1,2-diamines, which provided evidence of interchange.
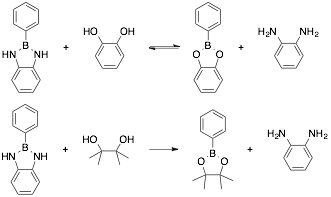
In addition to the synthetic work discussed above, we have been working towards understanding the dynamic behavior of boronic acids (or esters) and 1,2-phenylenediamines. Transamidation, or interchange, between diazaborole derivatives has been investigated using various phenyl boronic acid and ortho-phenylenediamine derivatives. The equilibration time is also long (>7 d). When benzodiazaborole was reacted with pinacol (an aliphatic diol) complete conversion was observed to the pinacol ester.
Solution studies concerning the dynamic covalent nature of the oxazaboroles and the relative stability compared to the well-established benzodioxaboroles were carried out using 1H NMR in CDCl3. The results of these studies revealed that oxazaboroles exhibit dynamic covalent nature. The reaction with dioxaborole (Y = O) reached a seemingly steady state much faster than diazaborole (Y = NH) (<5 min versus 7 d, respectively). Additionally, the stability of benzoxazaborole was also found to be similar to benzodioxaboroles and about an order of magnitude more stable than benzodiazaboroles.
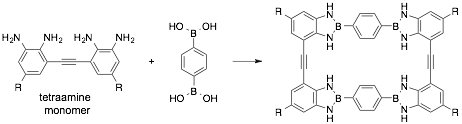
Diazaborole-based macrocycle: using the insight that was obtained in the studies described above, we synthesized a rectangular shaped diazaborole-based macrocycle from a tetraamine monomer (R = methyl) and phenylene diboronic acid. The monomer, which was available in a few steps from commercially available materials, was resynthesized having triethylene glycol chains [R = CO2Tg, Tg = -(CH2CH2O)3CH3] or ethylhexyl side chains, with anticipation of increasing the macrocycle’s solubility. Improved solubility of all intermediates was observed; however, we are still limited to solvents such as DMSO for the synthesis and characterization of oligomeric/macrocyclic products.
Impact on students: Four Master’s students and five undergraduate students have contributed to the above described work. Their work was presented at the ACS Southwest Regional Meeting (3 poster presentations) and the Texas Academy of Science annual meeting (2 poster and 2 oral presentations). PRF funds were used to support student travel and registration for the TAS meeting. We have also submitted four abstracts to the upcoming ACS Southwest Regional Meeting. Two of the Master’s students have graduated and are now enrolled in a PhD programs in chemistry. Three of the undergraduate students have graduated and are employed in various fields. One of them presented at the undergraduate research symposium, graduated with honors, and completed a Honors Thesis based on research supported by this grant. The funds form this grant have also been used to support one graduate student over the summer and one undergraduate student during the spring semester. The other students were supported through our Welch summer research program.