Reports: UNI156852-UNI1: Development of a Novel and Versatile Method for the Synthesis of Substituted Cyclobutanes
Michael R. Gesinski, PhD, Southwestern University
Substituted cyclobutanes have generated increasing interest in the fields of polymer, materials, and pharmaceutical sciences. For example, the strain associated with the reduced bond angle of cyclobutane rings make them useful reactive intermediates in organic synthesis and has served as active functional groups in stress-responsive polymers. In spite of this increasing interest, there is a paucity of methods for the synthesis of cyclobutanes, especially compared to other ring sizes. This grant has provided funding for research focused on the development of a novel method for the synthesis of substituted cyclobutanes using low-valent titanium (Kulinkovich) intermediates. It has also been discovered that valuable 1,4-diketones can also be synthesized using similar reaction conditions.
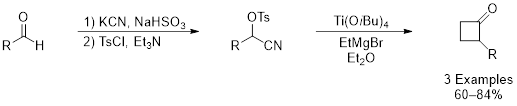
While many studies have explored the reaction of Kulinkovich reagents with esters, amides, and nitriles to form cyclopropanols, there have been no reports of reactions with dielectrophilic compounds. It was postulated that 1,2-dielectrophiles would react with these low-valent titanium species to afford substituted cyclobutane rings. To test this hypothesis, tosylated cyanohydrins were synthesized from commercially available aldehydes through a simple two-step process. When exposed to Kulinkovich conditions, the expected cyclobutanone was formed in 60% to 84% unoptimized yield, depending on the substrate. Further optimization of this reaction is necessary as well as exploration of substrate scope.
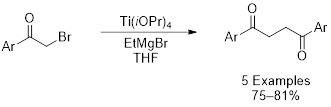
In an attempt to further expand this methodology, 2-bromoacetophenone was subjected to Kulinkovich conditions. Surprisingly, the reaction cleanly afforded a 1,4-diketone, the product of a dimerization reaction. These organic moieties serves as valuable synthetic building blocks because they are used as precursors to furans, thiophenes, and pyrroles. We have shown that this reaction likely proceeds through a carbon-based radical that is formed as the result of Ti(III) intermediates. Continued expansion of the substrate scope is underway, including aliphatic ketones and amides.
This grant has had a profound positive effect on both the primary investigator and the students that have participated in this project. Through this funding program, three undergraduate students and the PI were able to completely immerse themselves in this research program during eight weeks over the summer. Additionally, five other undergraduate researchers have benefited indirectly from this grant through the funding of supplies and laboratory equipment. Finally, the travel award in this grant has allowed the PI to present his work at the 2017 ACS National Meeting in San Francisco and allowed four students to present at the 2017 National Organic Symposium in Davis, CA. These experiences are transformative for students conducting research at primarily undergraduate institutions as it allows them to interact with a wide variety of scientists at the forefront of their fields.
Ultimately, this has laid the foundation for a sustainable research program in organic chemistry at Southwestern University and will expedite the publication of this fundamental scientific work. It is expected that this will lead to future successful grant applications from other funding organizations such as the National Science Foundation and the National Institute of Health.