Reports: UR455137-UR4: The Strength of Intramolecular Hydrogen Bonds in Fluoroorganic Molecules
Robert E. Rosenberg, Ph. D, Transylvania University
Introduction
The goal of this project is to determine the strengths of intramolecular hydrogen bonds, IMHB. To this end, we use a combination of computational chemistry, NMR spectroscopy, and synthesis.
Computational results
A large set of compounds has been studied computationally. Binding energies for the small subset of compounds studied in this report are shown in Table 1, below. It was found that three factors affected IMHB strength, 1) the size of the ring formed by F-C-(C)n-C-O-H and 2) the electron withdrawing/donating power of the carbons C(F) and C(OH), and the flexibility of the ring. The goal of the study is to test all three factors.
Table 1. Calculated binding energies of selected compounds
Name | IMHB energy (kcal/mol) |
2-fluorophenol | –2.80 |
2-(fluoromethyl)phenol | –1.42 |
2-(2-fluoroethyl)phenol | –1.25 |
8-fluoronaphthalen-1-ol | –3.72 |
9-fluoro-9H-fluoren-1-ol | –1.97 |
Approximated at CCSD(T)/aug-cc-pVTZ // MP2/aug-cc-pVDZ
Experimental methodology
NMR titration.
The main project in this study is using NMR spectroscopy to gather data from a titration experiment to measure binding data. Examination of the literature showed an unexpected variation in binding energies, making it hard to determine the quality of my student's data from last summer. As this methodology is the key to the project, I decided to redo a few of my student's results.
With the samples in hand, I also decided to do variable temperature work from 25° C to 45° C. The results are shown in Table 2, below. The data is in reasonable accord with the literature. While there is some disparity in the data for K, it is important to realize the ΔG is derived from the natural log of K. From this point of view, our calculated ΔH, ΔG values are very to the literature values.
Table 2, Binding constants (K) and thermodynamic constants
Compound | K (this work-student numbers in parentheses) | K (low) | K (high) | ΔH (this work) (kcal/mol) | ΔS (this work) (cal/mol K) |
4-fluorophenol | 270 (221) | 340a | 3500b | 5.8 (6.65b) | 8.6(6.1b) |
2-fluorophenol | 55.1 (55.7) | 53.5c | 254c | 5.2 | 9.7 |
2-methoxyphenol | 2.25 (2.4) | 0.83c | 4.1d | 3.8 (3.04d) | 11.3 (7.57d) |
2-chlorophenol | 18.7 | 2.4c | 5.5c |
|
|
2-bromophenol | 17.4 | 3.3c | 8.2c |
|
|
aGurka, Det al. J. Am. Chem. Soc., 1967, 89, 5957-5958. bLaurence, C.; Gal, J. F. "Lewis Basicity and Affinity Scales: Data and Measurement", Wiley, Chicester, UK 2010 cAbraham, M.A et al. J. Chem. Soc..F,araday Trans. I , 1987, 83, 2867-2881. dSpencer, J. N et al. J. Phys. Chem. 1975, 79, 2488-2493.
Synthesis
Part 1. Rigid molecules.
Both projects here are continuations from the work of previous summers.
A. Synthesis of 8-Fluoro-4-methyl-1-naphthol
This compound is known, but its IMHB strength has not been measured. Earlier, a student had followed the published procedure to get a mixture of 8-Fluoro-4-methyl-1-naphthol and 5-Fluoro-4-methyl-1-naphthol. This year, a student was able to separate the two isomers by column chromatography leading to a 20-30 mg of each isomer. This separation is better both qualitatively and quantitatively than the our previous attempt.
Takemura, et al. J. of Fluorine Chem. 2009, 130, 684-688.
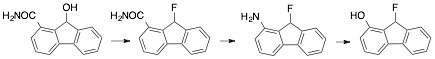
B. Synthesis of 9-fluoro-9H-fluoren-1-ol
This is another project started years ago. The goal is to form 9-fluoro-9H-fluoren-1-ol, from the known 9-hydroxy-9H-fluorene-1-carboxamide, of which we had made a few grams. The plan is convert the aromatic amine to an aniline, 1-amino-9H-fluoren-9-ol, using a Hofmann rearrangement. This would be followed by formation of the diazonium salt and decomposition to the phenol. Unfortunately, while my student was able to take benzamide and convert it to aniline, he was not able to convent 9-hydroxy-9H-fluorene-1-carboxamide to 1-amino-9H-fluoren-9-ol. Along the way, the same student was able to convert aniline to phenol, so we feel that we very close, at least in principle.
Part 2. Flexible molecules
A. Model studies
1. Protection and deprotection of a phenol.
Earlier we had successfully protected a phenol with a Boc group and could remove that group. However, in the actual synthesis, below, the Boc group did not work. So, we experimented with the MOM protecting group. Here, again we were successful in both protection and deprotection using 4-methylphenol as the model compound. Our second protecting group was methoxy. While there are several reports of anisole converting into phenol we had limited success in accomplishing this reaction.
2. Fluorination
Here we were able to directly convert both benzyl alcohol and 2-phenylethanol to the corresponding fluoro-compound using Deoxo-fluor. We also were able to convert benzyl alcohol to benzyl bromide using Br2 and PPh3 or PPh3 and CBr4. Then we were able to convert this bromide into the fluoride using TBAF.
B. Actual target molecules.
1. Synthesis of 2-(fluoromethyl)phenol
This project has continued to be frustrating. I think that we are forming the desired compound but that it is being destroyed under the reaction conditions. If the phenol is deprotonated, the fluorine is easily eliminated. The previous plan used the Boc protecting group. Here, we tried making both an OCH3 and MOM protection of the phenol. Neither seemed to work. However, we did run out of time with the MOM group, with the last experiments rushed.
2. Synthesis of 2-(2-fluoroethyl)phenol
Like most of these compounds, we envision two pieces. The formation of the fluoroethyl group could come from reduction of a carboxylic acid to the alcohol followed by conversion to fluorine using DeOxoFluor, which indeed worked quite well starting from 2-phenylacetic acid. The plan then was to repeat the synthesis starting with 2-(2-methoxyphenyl)acetic acid. This was converted to 1-(2-fluoroethyl)-2-methoxybenzene, but we were unable to convert the methoxy group to the phenol. The third approach, which we will continue next summer, is to first protect the phenol group with MOM. Then, the CH2COOH group will be reduced and fluorinated to CH2CH2F. Then the MOM group will be removed.
Training of undergraduates
This is the second year of the grant. I hired four students, one biology major, one biochemistry major and two chemistry majors. The biology major is a senior applying to medical schools. The other students are juniors with unclear plans.