Reports: DNI354834-DNI3: Upgrading Polyethylene: Copolymerization of Ethylene and Polar Vinyl Monomers Facilitated by Lewis Acid Appended Ni and Pd Catalysts
Loi H. Do, PhD, University of Houston
2017 PRF Annual Report
Project Background: During the prior PRF funding period (09/01/15 to 08/31/16), our group reported a new class of nickel phenoxyimine-polyethylene glycol olefin polymerization catalysts. We demonstrated that the addition of alkali ions to these mononickel complexes can provide up to a 20-fold enhancement in catalyst activity and significantly increase polymer molecular weight and branching compared to polymerizations performed in the absence of alkali ions. This past year (9/01/16 to 08/31/17), we developed sterically bulky variants of our first generation catalysts and investigated the effects of structural tuning on their catalyst activities and polymer morphologies. We have also prepared novel dinucleating ligand motifs to access structurally distinct classes of heterobimetallic catalysts that can overcome the limitations of existing catalyst systems.
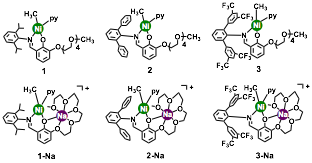
Bulky Nickel Phenoxyimine-PEG Catalysts. To investigate the influence of steric effects on the catalyst's properties, we synthesized a series of nickel phenoxyimine-PEG complexes with different N-aryl substituents (R = 2,6-diisopropylphenyl (1), 2,6-diphenylphenyl (2), and 2,6-bis(3,5-bis(trifluoromethyl)phenyl)phenyl (3)) (Chart 1). In general, we found that bulkier catalysts gave higher ethylene polymerization activity (3 > 2 > 1), with branches ranging from 20-90 per 10000 carbon atoms. The addition of Na+ salts (1 equiv.) to the nickel catalysts afforded the corresponding nickel-sodium complexes, 1-Na, 2-Na, and 3-Na. Theses heterobimetallic species are far more active catalysts compared to their parent mononickel species. For example, the turnover frequency (TOF) of 3-Na is about 330-fold greater than that of complex 1 in ethylene homopolymerization. Similar trends were observed when potassium salts were used instead of sodium. Interestingly, the heterobimetallic catalysts gave polymers with lower molecular weight than the monometallic catalysts. This observation is surprising because we had previously observed the opposite effect (i.e. addition of alkali ions lead to an increase in Mn). We hypothesize that differences in the supporting ligands of the nickel pre-catalysts (pyridine in 1 vs. triphenyphosphine in previous system) might play a role, but we are currently investigating further.
Chart 1.
Copolymerization studies were also performed using catalysts 3 and 3-Na. Similar to studies of ethylene homopolymerization, we observed that 3-Na is a far more efficient catalysts than 3 in the copolymerization of ethylene and non-polar 1-alkenes, such as 1-hexane and 1-heptene. For example, the TOFs of 3-Na is about 20-fold greater than that of 3 for ethylene/1-hexene copolymerization. We discovered that despite the increased catalyst activity of 3-Na compared to 3, it does not copolymerization ethylene with polar olefins such as methyl acrylate or vinyl acetate. Because these nickel phenoxyimine-PEG catalysts can yield narrowly-dispersed polyolefins with different morphologies, they might be useful for the preparation of designer polymers for specialized applications.
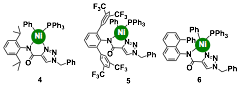
Triazolecarboxamidate Donors as Supporting Ligands. To expand the structural diversity of dinucleating ligands that are available for the preparation of bimetallic olefin polymerization catalysts, we wanted to design ligand platforms that have non-oxygen bridging donors (e.g. avoid alkoxide or phenoxide groups). Due to their ease of synthesis and potential to bind to multiple metal centers, we prepared novel ligands based on 1,2,3-trizaole-4-carboxamidate donors (Chart 2). These N,N-donors can readily be metallated with various nickel precursors to furnish complexes 4, 5, or 6. We showed by NMR spectroscopy, that the addition of zinc triflate to a solution of 4 in chloroform-d1 led to spectral changes that suggested the formation of Ni/Zn bimetallic species. We do not yet have structural characterization of this complex. The mononickel complexes 4-6, upon activation by treatment with Ni(COD)2 (COD = 1,5-cylcooctadiene), were active as catalysts for ethylene homopolymerization. As expected, the identities of their N-aryl substituents strongly influence their catalyst activities and polymer morphologies. Ongoing work is focused on the preparation of triazolecarboxamide ligands that have additional donor groups attached to the trizaole ring, which would provide a new series of dinucleating ligands to prepare well-defined heterobimetallic complexes.
Chart 2.
Palladium Catalyst Systems. Other research activities in the Do group are focused on the development of palladium olefin polymerization catalysts that also feature pendant Lewis acids. We have recently synthesized a new family of palladium phosphine-phosphinato complexes that have multiple PEG arms to chelate secondary metal ions. We have preliminary data to show that in the presence of alkali ions, the palladium catalysts are capable of copolymerization ethylene and methyl acrylate with high efficiency.
Impact of PRF Support. The funding provided by this ACS PRF-DNI grant has enabled our group to explore the concept of Lewis-acid assisted coordination insertion polymerization and to create novel synthetic platforms to test this mechanism. This grant supported several graduate, undergraduate, and high school students who took part in this project, which allowed them to acquire useful industrially relevant knowledge and experiences in catalyst design, organometallic chemistry, and polymer chemistry.