Reports: ND155652-ND1: Conversion of Alkynes to alpha-Branched Products via Propargylic Substitution
Jeremy A. May, PhD, University of Houston
Summary of proposed goals in the grant application:
Aim 1: Synthesize a library of Tp ligands with variations in sterics and electronics and then use them to synthesize an array of organometallic complexes based on preliminary results with privileged substrates.
Aim 2A: Synthesize key inter- and intramolecular substrates to test if the complexes replicate or exceed current catalysts
Aim 2B: Show propargylic substitution for substrates with aliphatic substation and form products with quaternary carbons.
Results for Aim 1:
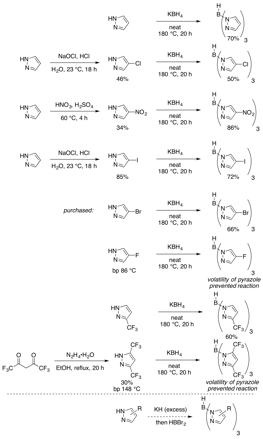
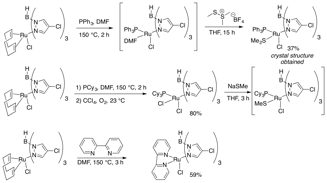
Much of this aim was accomplished during the first year of the grant period. We synthesized a library of ligands that vary electronically and present varied steric profiles (Figure 1). An exception as the fluorinated Tp ligands, where the volatility of the pyrazoles foiled the reaction. Consequently, we developed in this second year a new selective modification of trispyrazolylborate ligands while they are ligated to metals. This advance allows for rapid catalyst diversification to achieve optimal results (see below, Figure 3).
Figure 1: Ligand Synthesis
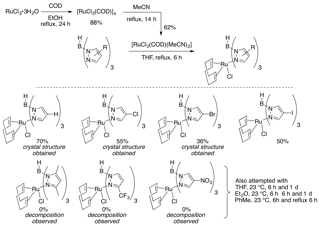
We have identified two strategies for synthesizing Ru(II)Tp complexes from the relatively cheap precursor RuCl3. The first reduces RuCl3in the presence of COD and then forms the bisacetonitrile complex, which is primed for substitution (Figure 2). Exposure of this complex to Tp provided the ligated metal.
Figure 2: Ligand Addition to Ruthenium Strategy 1
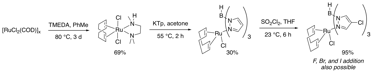
In a new strategy, RuCl2(COD) oligomer is treated with TMEDA to activate it for substitution (Figure 3). The incorporation of an unsubstituted Tp ligand offers rapid catalyst diversification from a single, late-stage intermediate. To date, we have identified conditions for selective chlorination, bromination, and iodination at the 4-position of the Tp ligand. These halogenations occur via electrophilic aromatic substitution. We also have promising preliminary results for the addition of fluoride to these ligands, which avoids the problems mentioned in Figure 1. We are also demonstrating that we can further functionalize these aryl halides, for example via cross-coupling reactions. The ability to modify a late stage intermediate is allowing us to rapidly search for desired catalytic activity.
Figure 3: Ligand Addition to Ruthenium Strategy 2
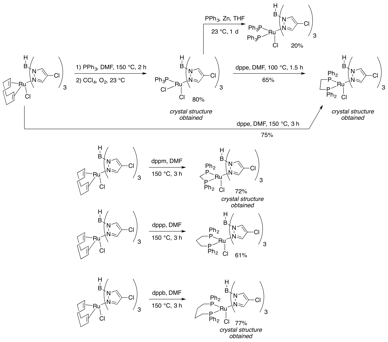
Based on indications that phosphine ligands may stabilize the 16-electron ruthenium active catalyst, we have also synthesized a number of phosphine-ligated ruthenium-Tp complexes (Figure 4). Both Ru(II) and Ru(III) complexes have been made, and monodentate or bidentate ligands incorporated.
Figure 4: Addition of Phosphines to the Complex
To further explore the substitution chemistry of these Tp complexes, the experiments in Figure 5 were made. In the first, a sulfur-ligated Me2S complex was isolated and characterized, and then we also demonstrated the addition of NaSMe to a Ru(III) PCy3 complex. Loss of the phosphine did not occur, which allowed the complex to remain monomeric. Lastly, a diamine ligand was added to see any changes in catalytic activity. A range of P,N and P,O ligands will also be incorporated. All synthesized complexes were analyzed by NMR, UV-vis, and X-ray diffraction spectroscopic techniques.
Figure 5: Additional Non-Phosphine Ligands
Results for Aim 2A:
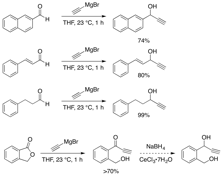
We have chosen to synthesize four simple, but representative, substrates to test the catalysts. First is a naphthylated propargylic alcohol that is easy to monitor in the reaction and is a privileged substrate for the propargylation of nucleophiles to serve as the positive control (Figure 6). A styrenyl alcohol has also been synthesized as a mechanistic indicator, as cation formation would lead to allylic mixtures of products, but a true allenylidene intermediate (which is proposed for our catalysts) allows only the propargylic substitution. An aliphatic substrate has been synthesized for intermolecular reactions, and a benzylic alcohol has been prepared to demonstrate intramolecular reactivity.
Figure 6: Substrate Synthesis
Results for Aim 2B:
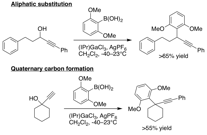
We have begun our tests with our catalyst library and the substrates above. In short, several of our monomeric complexes replicate the activity of the structurally more complex dimeric complexes developed by Hidai and utilized by Nishibayashi, so we view the simplicity of our complexes as an advantage. Unfortunately, the limitations in substrate scope of these Ru complexes remains similar to that for the dimeric complexes. Nevertheless, in the course of these studies, we made an exciting new discovery in propargylic substitutions that completely accomplishes the main two goals of aim 2 of this grant: (A) tolerate aliphatic substitution in substrates and (B) form quaternary compounds. In control experiments comparing the activity of ruthenium complexes to Lewis acid catalysis, we have found that gallium is able to perform regioselective propargylic substitution for both goals (A) and (B) (Figure 7). While this avenue of catalysis was unanticipated, it does serendipitously solve the problems at hand via research and personnel supported by this PRF grant.
Figure 7: Gallium catalysis
Impact on the careers of the PI and students supported:
The award has had a significant and beneficial impact on the PI's career in allowing him to explore a new field of research, which differs not only from prior independent efforts but also from his PhD and postdoctoral training. Moreover, the catalytic discoveries during the grant period now support his synthetic efforts in a synergistic fashion that will contribute to his research program for years. Additionally, multiple graduate students financially supported in this research will have their contributions incorporated into their PhD theses. They have experienced all aspects of scientific discovery from hypothesis-based research to serendipitous discovery. They have also benefited from exploring the interdisciplinary intersection of inorganic synthesis with synthetic organic methods development, which would not have been possible without the award.
Conclusion
The funding from the PRF has allowed the synthesis of a library of novel ruthenium catalysts, whose novelty merits their publication independent of their catalytic activity. An efficient method of functionalizing the ligands while attached to metals has been developed, which allows for rapid catalyst diversification to optimize reactivity. Thus the first Aim of the grant has been accomplished.
Key substrates for the exploration of catalytic activity have been synthesized and tested. The goals in the second Aim of the grant have been realized via serendipitous discovery of gallium-catalyzed propargylic substitution during control experiments.