Reports: DNI1056997-DNI10: Cu-Containing Bimetallic Nanoparticles for Electrocatalytic CO2 Reduction into Fuels
Hailiang Wang, Yale University
The research project is mainly focused on the materials chemistry of Cu-containing bimetallic and metal/oxide heterostructured nanomaterials as catalysts for electrochemical CO2 reduction reactions, with the aims to develop electrocatalysts that are able to selectively and rapidly convert CO2 to useful products as well as to understand the metal-metal or metal-oxide interactions and their influences on electrocatalytic reactivity.
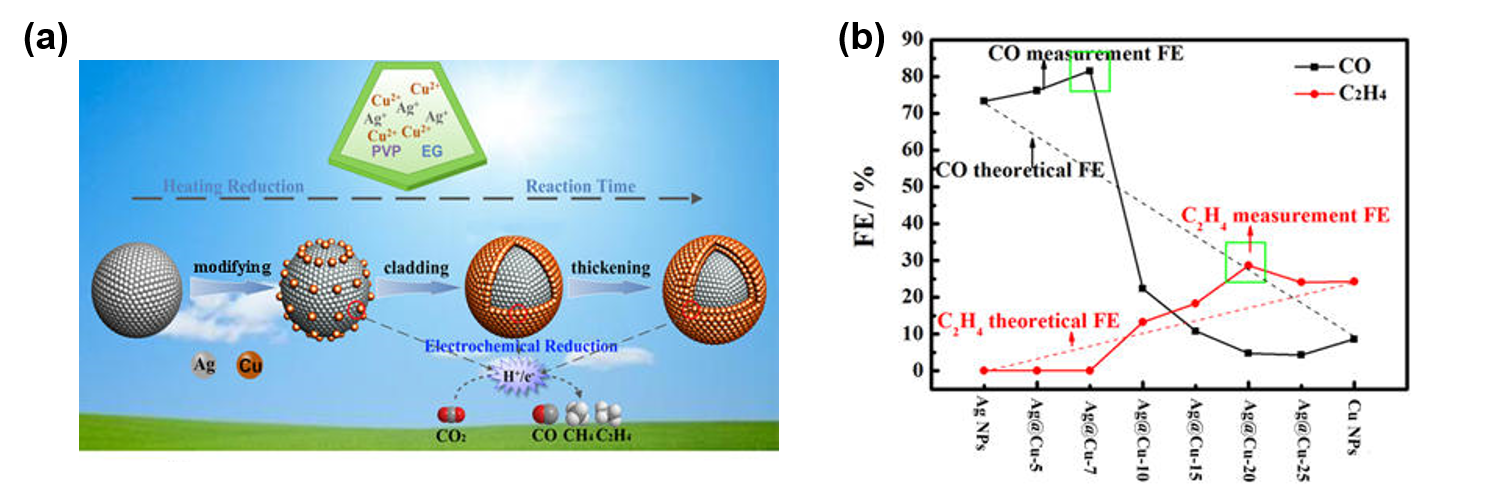
We first studied a Cu-Ag bimetallic nanoparticle system. The nanoparticles were synthesized by a polyol method, in which a mixture of Cu2+ and Ag+ were reduced by ethylene glycol (EG) in the presence of polyvinylpyrrolidone (PVP). Depending on the reaction time, the structure of the obtained Cu-Ag nanoparticles changes from Ag nanoparticles decorated with discontinuous Cu patches on the surface to Ag@Cu core@shell nanoparticles (Figure 1a). The interactions between the Ag component and the Cu component in the material structure generate interesting electrocatalytic properties toward CO2 reduction. In the Ag-rich composition range, the intrinsic catalytic behavior of Ag is improved to give even higher selectivity toward production of CO (Figure 1b). In the Cu-rich range, Ag modifies the catalytic selectivity of Cu, achieving higher Faradaic efficiency (FE) for CO2 reduction to ethylene (C2H4). This work has been published as a research article in J. Phys. Chem. C.
Figure 1. (a) Schematic illustration of the structural evolution of Ag-Cu bimetallic nanoparticles as the synthesis reaction time increases. (b) Composition-dependent product selectivity for the Ag-Cu materials as electrocatalysts for CO2 reduction at -1.06 V vs RHE in 0.1 M aqueous KHCO3 (pH 6.8) saturated with CO2.
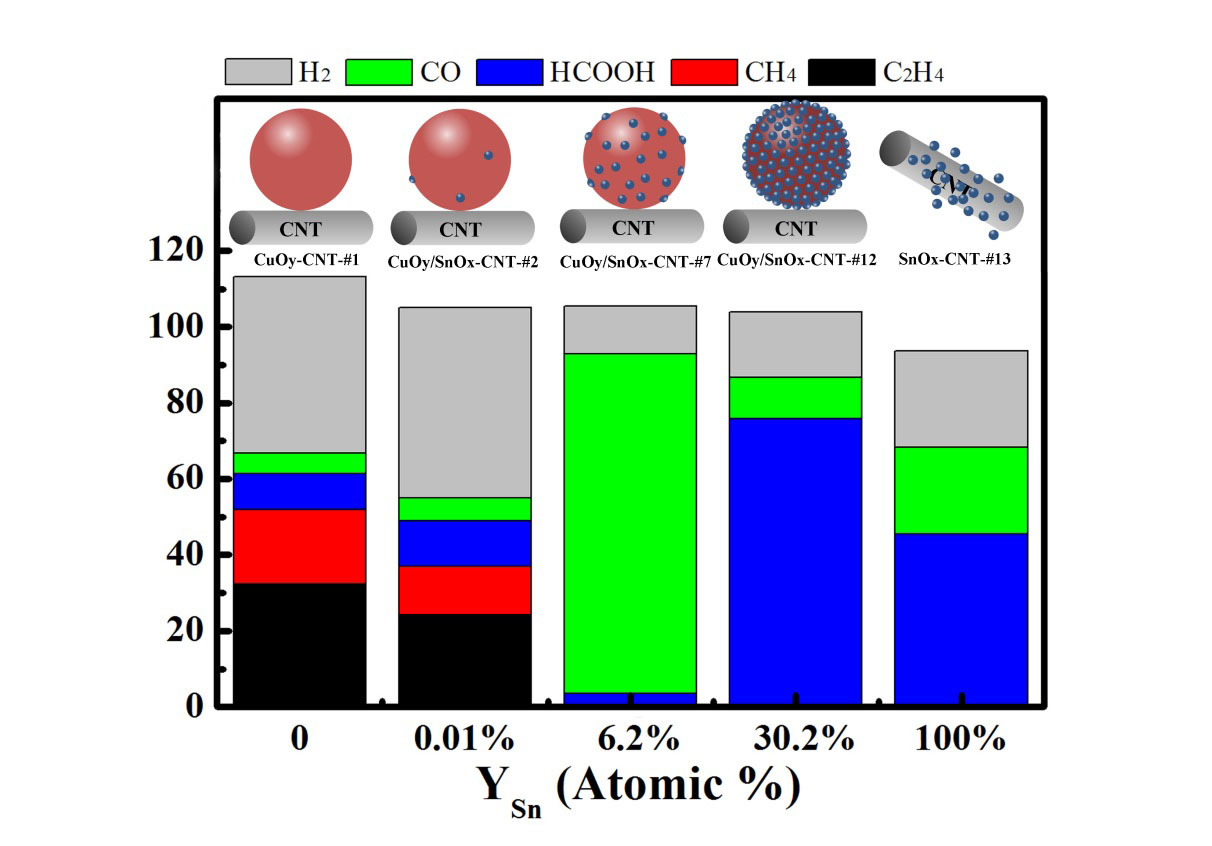
We also studied a Cu-SnOx heterostructured nanoparticle system, using mildly oxidized carbon nanotubes (CNTs) as the catalyst support. By adjusting the Cu/Sn ratio in the catalyst material structure, we can tune the products of the CO2 electrocatalytic reduction reaction from hydrocarbon-favorable to CO-selective to formic-acid-dominant (Figure 2). In the Cu-rich regime, SnOx dramatically alters the catalytic behavior of Cu. The Cu/SnOx-CNT catalyst containing 6.2% of SnOx converts CO2 to CO with a high FE of 89% and a jCO of 11.3 mA·cm-2 at -0.99 V vs RHE, in stark contrast to the Cu-CNT catalyst on which ethylene and methane are the main products for CO2 reduction. In the Sn-richer regime, Cu modifies the catalytic properties of SnOx. The Cu/SnOx-CNT catalyst containing 30.2% of SnOx reduces CO2 to formic acid with a FE of 77% and a jHCOOH of 4.0 mA·cm-2 at -0.99 V, outperforming the SnOx-CNT catalyst which only converts CO2 to formic acid in a FE of 48%. This work, which has been published as a research article in ACS Appl. Mater. Interfaces, represents one of the first systematic study on composition-dependent influences of metal-oxide interactions on electrocatalytic CO2 reduction.
Figure 2. Correlations between material structures and product distributions for composition-tunable Cu/SnOx-CNT materials as electrocatalysts for CO2 reduction.
These results have brought us new knowledge of utilizing metal-metal and metal-oxide interactions to improve electrocatalysis for the important CO2 conversion reactions. We have developed Cu-containing nanoparticle electrocatalysts with tunable product selectivity for the CO2 reduction reactions, and have gained preliminary insights into how Cu interacts electronically with another metal or a metal oxide and manifests different catalytic properties.
Our research in the following year will answer the remaining questions in this project. We would like to explore how metal-metal and metal-oxide interactions can be utilized to achieve more active and stable electrochemical CO2 conversion, in addition to the product selectivity that we focused on investigating in the past year. Our current understanding has mainly been on electronic interactions between two components within a material structure, we would like to examine the possibilities of other cooperation modes, such as reaction intermediate co-stabilization and hydrogen spillover. We anticipate that at the end of this project we will have a toolbox for designing advanced electrocatalysts for CO2 reduction reactions by coordinating metal-metal and metal-oxide interactions.